Anat Cell Biol.
2014 Sep;47(3):180-187. 10.5115/acb.2014.47.3.180.
Localization of S-100 proteins in the testis and epididymis of poultry and rabbits
- Affiliations
-
- 1Department of Histology and Cytology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
- 2Department of Anatomy, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt. mshoaib882004@yahoo.com
- KMID: 1882593
- DOI: http://doi.org/10.5115/acb.2014.47.3.180
Abstract
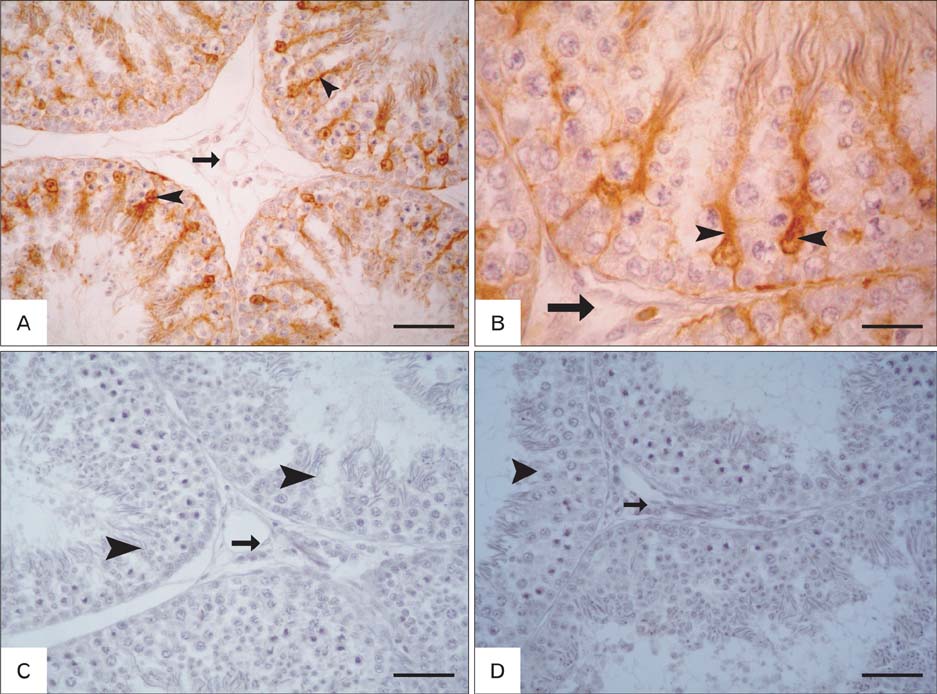
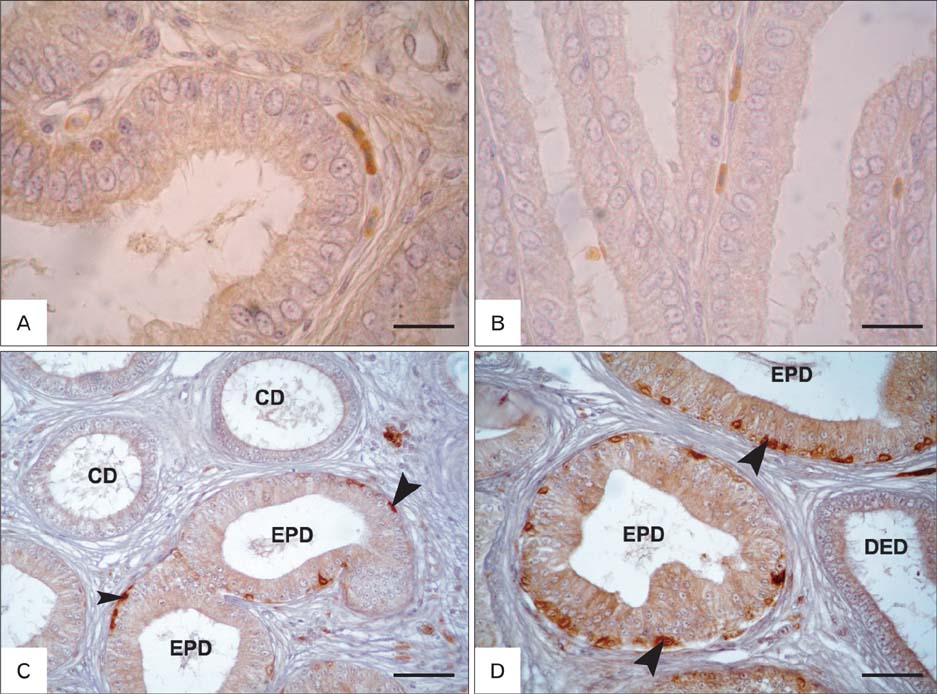
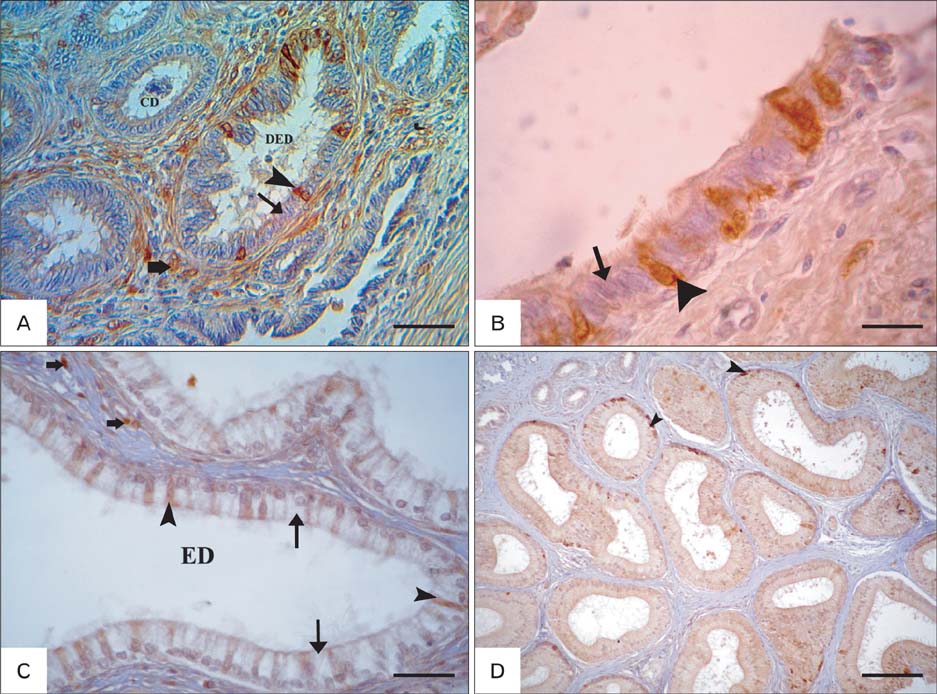
- The present investigation was conducted to demonstrate S-100 protein in the testis and epididymis of adult chickens, Sudani ducks, pigeons, and rabbits. This study may represent the first indication for the presence of S-100 in the male reproductive organs of these species and might therefore serve as a milestone for further reports. In the testis of chickens, pigeons and rabbits, intense S-100 was seen in Sertoli cells. S-100 was also seen in the endothelial lining of blood vessels in rabbit testis. On the contrary, no S-100 reaction was detected in the Sertoli cells of Sudani ducks. In epididymis, the localization of S-100 had varied according to species studied; it was seen in the basal cells (BC) of epididymal duct in duck, non-ciliated cells of the distal efferent ductules in pigeons and ciliated cells of the efferent ductules and BC of rabbit epididymis. Conversely, S-100 specific staining was not detected in the epithelial lining of the rooster and pigeon epididymal duct as well as the principal cells of the rabbit epididymis. In conclusion, the distribution of the S-100 proteins in the testis and epididymis might point out to its roles in the male reproduction.
Keyword
MeSH Terms
Figure
Reference
-
1. Tingari MD. On the structure of the epididymal region and ductus deferens of the domestic fowl (Gallus domesticus). J Anat. 1971; 109(Pt 3):423–435.2. Budras KD, Sauer T. Morphology of the epididymis of the cock (Gallus domesticus) and its effect upon the steroid sex hormone synthesis Ontogenesis, morphology and distribution of the epididymis. Anat Embryol (Berl). 1975; 148:175–196.3. Hess RA, Thurston RJ, Biellier HV. Morphology of the epididymal region and ductus deferens of the turkey (Meleagris gallopavo). J Anat. 1976; 122:241–252.4. Stefanini MA, Orsi AM, Gregório EA, Viotto MJ, Baraldi-Artoni SM. Morphologic study of the efferent ductules of the pigeon (Columba livia). J Morphol. 1999; 242:247–255.5. Aire TA, Soley JT. The surface features of the epithelial lining of the ducts of the epididymis of the ostrich (Struthio camelus). Anat Histol Embryol. 2000; 29:119–126.6. Abd-Elmaksoud A, Sayed-Ahmed A, Mohamed SE, Mohamed K, Marei HE. Morphological and glycohistochemical studies on the epididymal region of the Sudani duck (Cairina moschata). Res Vet Sci. 2009; 86:7–17.7. Tingari MD, Lake PE. Histochemical localization of glycogen, mucopolysaccharides, lipids, some oxidative enzymes and cholinesterases in the reproductive tract of the male fowl (Gallus domesticus). J Anat. 1972; 112(Pt 2):273–287.8. Clulow J, Jones RC. Studies of fluid and spermatozoal transport in the extratesticular genital ducts of the Japanese quail. J Anat. 1988; 157:1–11.9. Janssen SJ, Bunick D, Finnigan-Bunick C, Chen YC, Hess R, Bahr JM. Morphology and function of rooster efferent ductule epithelial cells in culture. Tissue Cell. 1998; 30:554–561.10. Janssen SJ, Kirby JD, Hess RA, Rhoads M, Bunick D, Bailey KL, Parsons CM, Wang H, Bahr JM. Identification of epididymal stones in diverse rooster populations. Poult Sci. 2000; 79:568–574.11. Bahr JM, Dalponte M, Janssen S, Bunick D, Nakai M. Ion transporters for fluid reabsorption in the rooster (Gallus domesticus) epididymal region. Anim Reprod Sci. 2006; 95:331–337.12. Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965; 19:739–744.13. Donato R. S-100 proteins. Cell Calcium. 1986; 7:123–145.14. Zimmer DB, Cornwall EH, Landar A, Song W. The S100 protein family: history, function, and expression. Brain Res Bull. 1995; 37:417–429.15. Donato R. Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta. 1999; 1450:191–231.16. Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001; 33:637–668.17. Schäfer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. Trends Biochem Sci. 1996; 21:134–140.18. Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998; 11:383–397.19. Heizmann CW, Fritz G, Schafer BW. S100 proteins: structure, functions and pathology. Front Biosci. 2002; 7:d1356–d1368.20. Amselgruber W, Sinowatz F, Schams D, Lehmann M. S-100 protein immunoreactivity in bovine testis. Andrologia. 1992; 24:231–235.21. Amselgruber WM, Sinowatz F, Erhard M. Differential distribution of immunoreactive S-100 protein in mammalian testis. Histochemistry. 1994; 102:241–245.22. Abd-Elmaksoud A. Morphological, glycohistochemical, and immunohistochemical studies on the embryonic and adult bovine testis [dissertation]. Munich: Institute of Veterinary Anatomy II, Faculty of Veterinary Medicine;2005.23. Alkafafy M. Glycohistochemical, immunohistochemical and ultrastructural studies of the bovine epididymis [dissertation]. Munich: Institute of Veterinary Anatomy II, Faculty of Veterinary Medicine, LMU;2005.24. Michetti F, Lauriola L, Rende M, Stolfi VM, Battaglia F, Cocchia D. S-100 protein in the testis. An immunochemical and immunohistochemical study. Cell Tissue Res. 1985; 240:137–142.25. Czykier E, Sawicki B, Zabel M. S-100 protein immunoreactivity in mammalian testis and epididymis. Folia Histochem Cytobiol. 2000; 38:163–166.26. Cruzana BC, Hondo E, Kitamura N, Matsuzaki S, Nakagawa M, Yamada J. Differential localization of immunoreactive alpha- and beta-subunits of S-100 protein in feline testis. Anat Histol Embryol. 2000; 29:83–86.27. Cruzana MB, Budipitojo T, De Ocampo G, Sasaki M, Kitamura N, Yamada J. Immunohistochemical distribution of S-100 protein and subunits (S100-alpha and S100-beta) in the swamp-type water buffalo (Bubalus bubalis) testis. Andrologia. 2003; 35:142–145.28. Alkafafy M, Elnasharty M, Sayed-Ahmed A, Abdrabou M. Immunohistochemical studies of the epididymal duct in Egyptian water buffalo (Bubalus bubalis). Acta Histochem. 2011; 113:96–102.29. Girod C, Durand N, Raccurt M. A comparative study of immunocytochemical localization of S-100 protein in the monkey (Macaca irus) and the albino rat testes. Biomed Res. 1986; 7:333–337.30. Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987; 57:489–498.31. Sugimura M, Miura M, Suzuki Y, Atoji Y. S-100 immunoreactive cells in non-nervous duck tissues. Avian Pathol. 1989; 18:503–510.32. Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981; 29:577–580.33. Czykier E, Sawicki B, Zabel M. Immunocytochemical localization of S-100 protein in the European bison testis and epididymis. Folia Histochem Cytobiol. 1999; 37:83–84.34. Alkafafy M. Some immunohistochemical studies on the epididymal duct in the donkey (Equus asinus). J Vet Anat. 2009; 2:23–40.35. Alkafafy M, Rashed R, Emara S, Nada M, Helal A. Histological and immunohistochemical studies on the epididymal duct in the dromedary camel (Camelus dromedarius). Anat Cell Biol. 2011; 44:284–294.