J Periodontal Implant Sci.
2015 Jun;45(3):101-110. 10.5051/jpis.2015.45.3.101.
Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner
- Affiliations
-
- 1Department of Molecular Genetics, School of Dentistry and Dental Research Institute, Seoul National University, Seoul, Korea. gskim@snu.ac.kr, baekjh@snu.ac.kr
- 2Department of Anatomy, Faculty of Dentistry, Mahidol University, Bangkok, Thailand.
- 3Department of Pharmacology, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
- KMID: 1824939
- DOI: http://doi.org/10.5051/jpis.2015.45.3.101
Abstract
- PURPOSE
Sclerostin, an inhibitor of Wnt/beta-catenin signaling, exerts negative effects on bone formation and contributes to periodontitis-induced alveolar bone loss. Recent studies have demonstrated that serum sclerostin levels are increased in diabetic patients and that sclerostin expression in alveolar bone is enhanced in a diabetic periodontitis model. However, the molecular mechanism of how sclerostin expression is enhanced in diabetic patients remains elusive. Therefore, in this study, the effect of hyperglycemia on the expression of sclerostin in osteoblast lineage cells was examined.
METHODS
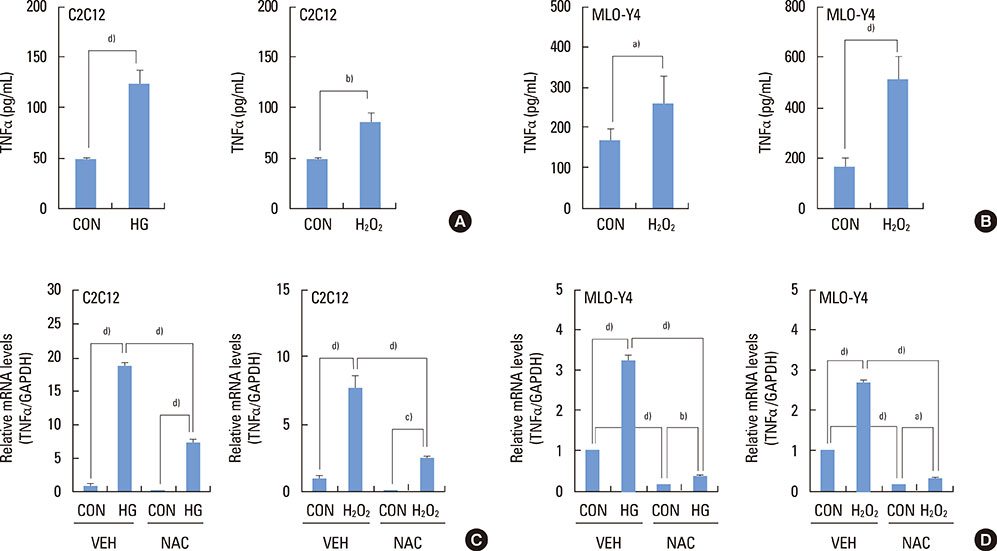
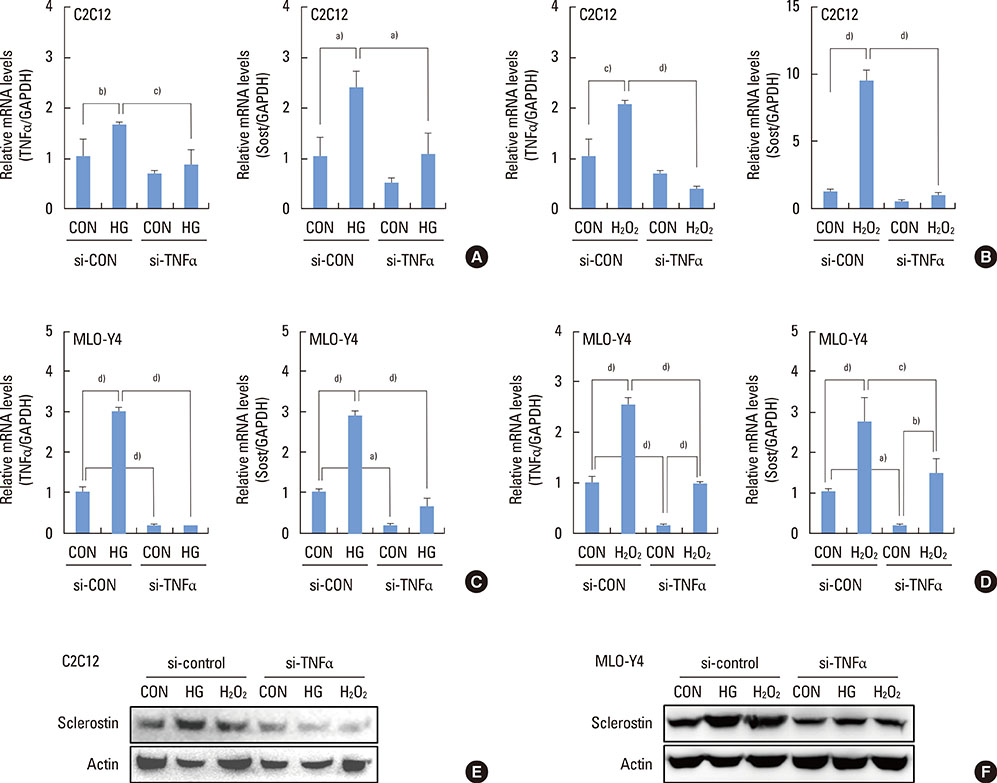
C2C12 and MLO-Y4 cells were used in this study. In order to examine the effect of hyperglycemia, the glucose concentration in the culture medium was adjusted to a range of levels between 40 and 100 mM. Gene expression levels were examined by quantitative reverse transcription-polymerase chain reaction and Western blot assays. Top-Flash reporter was used to examine the transcriptional activity of the beta-catenin/lymphoid enhanced factor/T-cell factor complex. Tumor necrosis factor-alpha (TNFalpha) protein levels were examined with the enzyme-linked immunosorbent assay. The effect of reactive oxygen species on sclerostin expression was examined by treating cells with 1 mM H2O2 or 20 mM N-acetylcysteine.
RESULTS
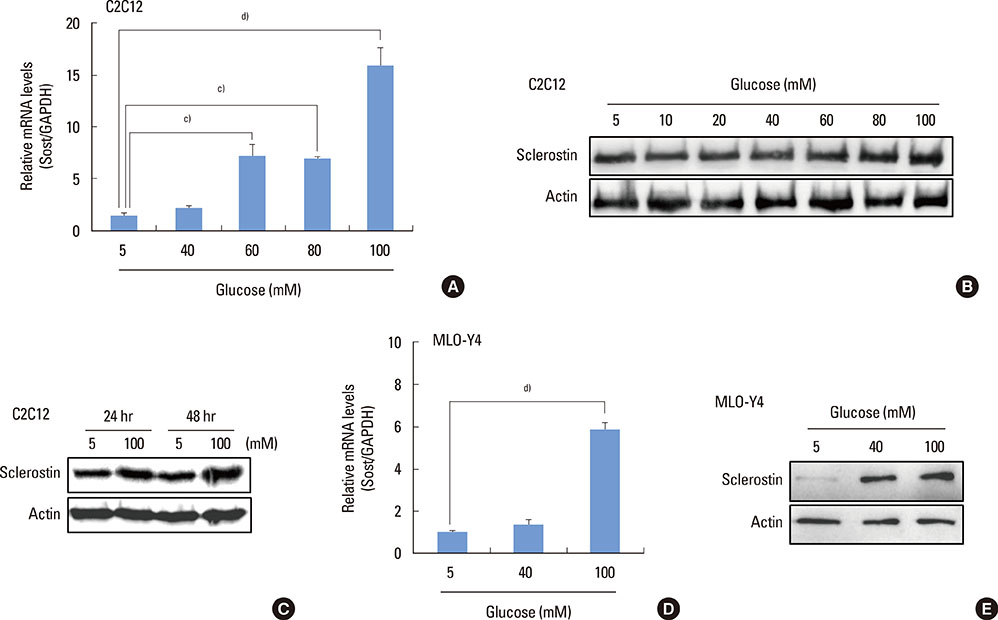
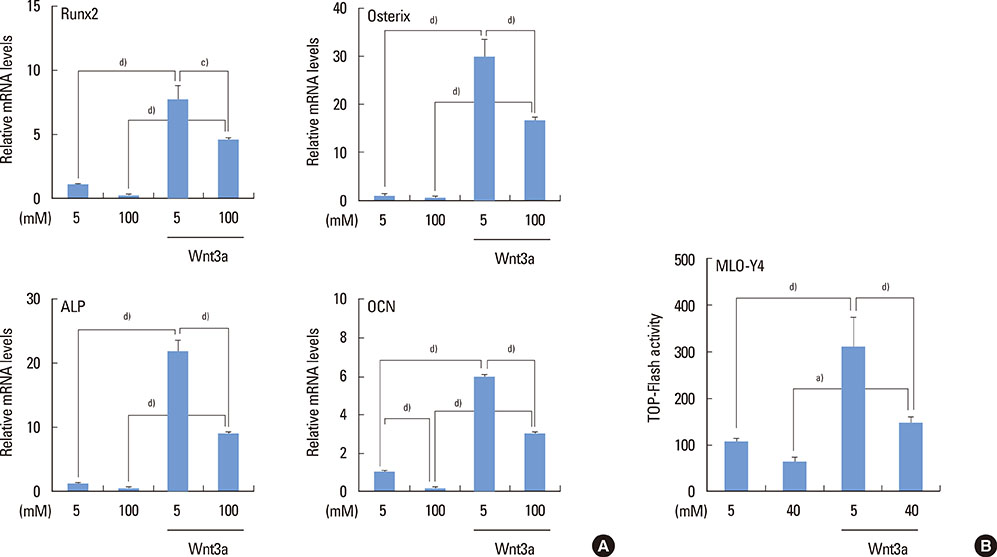
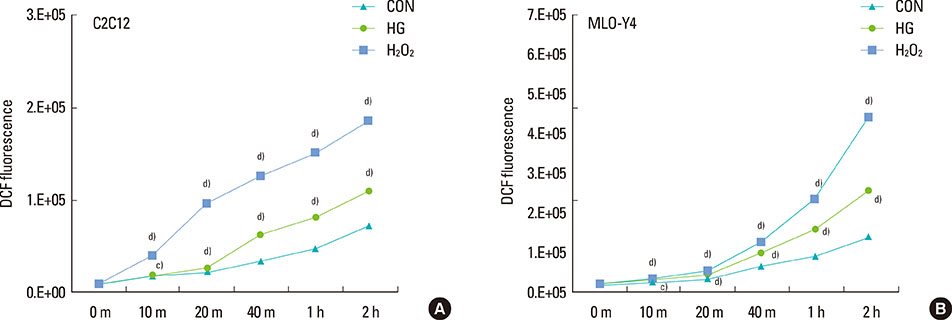
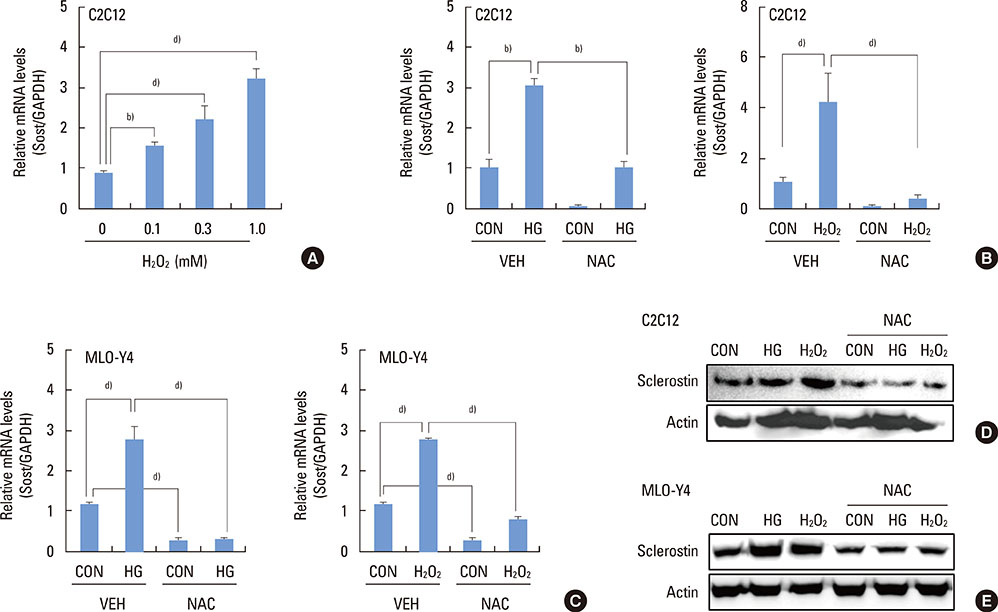
The high glucose treatment increased the mRNA and protein levels of sclerostin. High glucose suppressed Wnt3a-induced Top-Flash reporter activity and the expression levels of osteoblast marker genes. High glucose increased reactive oxygen species production and TNFalpha expression levels. Treatment of cells with H2O2 also enhanced the expression levels of TNFalpha and sclerostin. In addition, N-acetylcysteine treatment or knockdown of TNFalpha attenuated high glucose-induced sclerostin expression.
CONCLUSIONS
These results suggest that hyperglycemia increases sclerostin expression via the enhanced production of reactive oxygen species and TNFalpha.
MeSH Terms
-
Acetylcysteine
Alveolar Bone Loss
Blotting, Western
Enzyme-Linked Immunosorbent Assay
Gene Expression
Glucose
Humans
Hyperglycemia*
Necrosis*
Osteoblasts
Osteogenesis
Oxygen*
Periodontitis
Reactive Oxygen Species
RNA, Messenger
Tumor Necrosis Factor-alpha
Acetylcysteine
Glucose
Oxygen
RNA, Messenger
Reactive Oxygen Species
Tumor Necrosis Factor-alpha
Figure
Cited by 1 articles
-
Skeletal Fragility in Type 2 Diabetes Mellitus
Jakob Starup-Linde, Katrine Hygum, Bente Lomholt Langdahl
Endocrinol Metab. 2018;33(3):339-351. doi: 10.3803/EnM.2018.33.3.339.
Reference
-
1. Wu YY, Xiao E, Graves DT. Diabetes mellitus related bone metabolism and periodontal disease. Int J Oral Sci. 2015; Forthcoming.
Article2. Inaba M, Okuno S, Kumeda Y, Yamakawa T, Ishimura E, Nishizawa Y. Increased incidence of vertebral fracture in older female hemodialyzed patients with type 2 diabetes mellitus. Calcif Tissue Int. 2005; 76:256–260.
Article3. Epstein S, Leroith D. Diabetes and fragility fractures - a burgeoning epidemic? Bone. 2008; 43:3–6.
Article4. Lu H, Kraut D, Gerstenfeld LC, Graves DT. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology. 2003; 144:346–352.
Article5. Katayama Y, Akatsu T, Yamamoto M, Kugai N, Nagata N. Role of nonenzymatic glycosylation of type I collagen in diabetic osteopenia. J Bone Miner Res. 1996; 11:931–937.
Article6. Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008; 1126:166–172.7. Yamamoto T, Ozono K, Miyauchi A, Kasayama S, Kojima Y, Shima M, et al. Role of advanced glycation end products in adynamic bone disease in patients with diabetic nephropathy. Am J Kidney Dis. 2001; 38:S161–S164.
Article8. Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007; 40:1144–1151.
Article9. Roy B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes. 2013; 4:101–113.
Article10. Morikawa D, Nojiri H, Saita Y, Kobayashi K, Watanabe K, Ozawa Y, et al. Cytoplasmic reactive oxygen species and SOD1 regulate bone mass during mechanical unloading. J Bone Miner Res. 2013; 28:2368–2380.
Article11. Schröder K. NADPH oxidases in bone homeostasis and osteoporosis. Cell Mol Life Sci. 2015; 72:25–38.
Article12. Mackenzie RM, Salt IP, Miller WH, Logan A, Ibrahim HA, Degasperi A, et al. Mitochondrial reactive oxygen species enhance AMP-activated protein kinase activation in the endothelium of patients with coronary artery disease and diabetes. Clin Sci (Lond). 2013; 124:403–411.
Article13. Galli C, Piemontese M, Lumetti S, Manfredi E, Macaluso GM, Passeri G. The importance of WNT pathways for bone metabolism and their regulation by implant topography. Eur Cell Mater. 2012; 24:46–59.
Article14. Burgers TA, Williams BO. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone. 2013; 54:244–249.
Article15. Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005; 19:1842–1844.
Article16. Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007; 22:1317–1328.
Article17. Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV, et al. Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res. 2013; 28:2347–2356.
Article18. Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, et al. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB J. 2015; Forthcoming.
Article19. Chen H, Xu X, Liu M, Zhang W, Ke HZ, Qin A, et al. Sclerostin antibody treatment causes greater alveolar crest height and bone mass in an ovariectomized rat model of localized periodontitis. Bone. 2015; 76:141–148.
Article20. Baek K, Hwang HR, Park HJ, Kwon A, Qadir AS, Ko SH, et al. TNF-α upregulates sclerostin expression in obese mice fed a high-fat diet. J Cell Physiol. 2014; 229:640–650.
Article21. Ardawi MS, Akhbar DH, Alshaikh A, Ahmed MM, Qari MH, Rouzi AA, et al. Increased serum sclerostin and decreased serum IGF-1 are associated with vertebral fractures among postmenopausal women with type-2 diabetes. Bone. 2013; 56:355–362.
Article22. García-Martín A, Rozas-Moreno P, Reyes-García R, Morales-Santana S, García-Fontana B, García-Salcedo JA, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012; 97:234–241.
Article23. Neumann T, Hofbauer LC, Rauner M, Lodes S, Kästner B, Franke S, et al. Clinical and endocrine correlates of circulating sclerostin levels in patients with type 1 diabetes mellitus. Clin Endocrinol (Oxf). 2014; 80:649–655.
Article24. Jun JH, Lee SH, Kwak HB, Lee ZH, Seo SB, Woo KM, et al. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J Cell Biochem. 2008; 103:1246–1255.
Article25. Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000; 20:8783–8792.
Article26. Kim JH, Lee DE, Woo GH, Cha JH, Bak EJ, Yoo YJ. Osteocytic sclerostin expression in alveolar bone in diabetic rats with ligature induced-periodontitis. J Periodontol. 2015; 1–14.27. Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015; 461:193–199.
Article28. Ahrens B. Antibodies in metabolic diseases. N Biotechnol. 2011; 28:530–537.
Article29. Venieratos PD, Drossopoulou GI, Kapodistria KD, Tsilibary EC, Kitsiou PV. High glucose induces suppression of insulin signalling and apoptosis via upregulation of endogenous IL-1beta and suppressor of cytokine signalling-1 in mouse pancreatic beta cells. Cell Signal. 2010; 22:791–800.
Article30. David JP, Schett G. TNF and bone. Curr Dir Autoimmun. 2010; 11:135–144.
Article31. Kawai VK, Stein CM, Perrien DS, Griffin MR. Effects of anti-tumor necrosis factor α agents on bone. Curr Opin Rheumatol. 2012; 24:576–585.
Article32. Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000; 141:3956–3964.33. Nakase T, Takaoka K, Masuhara K, Shimizu K, Yoshikawa H, Ochi T. Interleukin-1 beta enhances and tumor necrosis factor-alpha inhibits bone morphogenetic protein-2-induced alkaline phosphatase activity in MC3T3-E1 osteoblastic cells. Bone. 1997; 21:17–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Institutions, Correspondence, Figures & Legends Correction. Hyperglycemia increases the expression levels of sclerostin in a reactive oxygen species- and tumor necrosis factor-alpha-dependent manner
- Role of Reactive Oxygen Species in Cell Death Pathways
- Mycobacterium tuberculosis Induces the Production of Tumor Necrosis Factor-alpha, Interleukin-6, and CXCL8 in Pulmonary Epithelial Cells Through Reactive Oxygen Species-dependent Mitogen-activated Protein Kinase Activation
- Rutin inhibits osteoclast formation by decreasing reactive oxygen species and TNF-alpha by inhibiting activation of NF-kappaB
- Shikonin Isolated from Lithospermum erythrorhizon Downregulates Proinflammatory Mediators in Lipopolysaccharide-Stimulated BV2 Microglial Cells by Suppressing Crosstalk between Reactive Oxygen Species and NF-kappaB