Platelet to lymphocyte ratio as a prognostic factor for epithelial ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Faculty of Medicine Vajira Hospital, University of Bangkok Metropolis, Bangkok, Thailand. siriwanonco@yahoo.com
- KMID: 1810124
- DOI: http://doi.org/10.3802/jgo.2012.23.4.265
Abstract
OBJECTIVE
To determine whether preoperative platelets to lymphocyte ratio (PLR) could predict disease stage, surgical outcome, and survival in patients with epithelial ovarian cancer (EOC).
METHODS
Medical records of EOC patients who had surgery between January 2004 and December 2010 were reviewed. Clinicopathological and complete blood count data were collected. The optimal predictive value of PLR to predict advanced stage, suboptimal surgery, and survival was determined and compared with those of thrombocytosis (> or =400,000 cells/mm3) and neutrophil to lymphocyte ratio (NLR) > or =2.6.
RESULTS
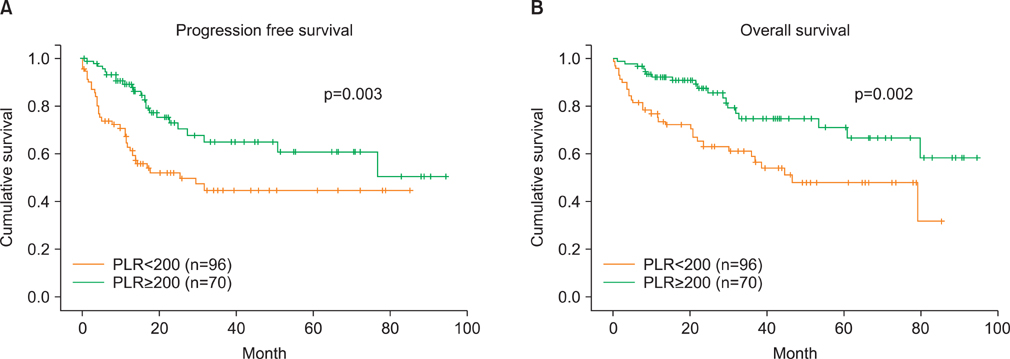
A total of 166 EOC patients were included in the study. PLR of 200 yielded better predictive values than those of thrombocytosis and NLR > or =2.6. The area under curve (AUC), sensitivity, specificity, positive and negative predictive values, and accuracy of PLR to predict advanced stage were: 0.66, 59.0%, 72.7%, 65.7%, 66.7%, and 66.3%, respectively. The corresponding values to predict suboptimal surgery were: 0.70, 70.0%, 69.8%, 50.0%, 84.4%, and 69.9%. The patients who had PLR> or =200 had significantly shorter progression-free and overall survivals than those with PLR<200. Stage, grade, surgical outcome, thrombocytosis, and PLR were significant prognostic factors for survivals by univariable analyses while only stage remained significant by multivariable analysis.
CONCLUSION
PLR had potential clinical value in predicting advanced stage disease or suboptimal surgery. PLR was a better prognostic indicator for survivals of EOC patients compared to thrombocytosis or NLR>2.6.
MeSH Terms
Figure
Cited by 3 articles
-
Platelet to lymphocyte ratio (PLR) retains independent prognostic significance in advanced stage marginal zone lymphoma patients treated with rituximab, cyclophosphamide, vincristine, and prednisone combination chemotherapy (R-CVP): Consortium for Improving Survival of Lymphoma trial
Jeongkuk Seo, Won Seog Kim, Jin Seok Kim, Seok Jin Kim, Jae Hoon Lee, Jun Shik Hong, Gyeong-Won Lee, Sung Yong Oh, Ji-Hyun Lee, Dok Hyun Yoon, Won-Sik Lee, Hyo Jung Kim, Jae-Yong Kwak, Hye Jin Kang, Jae-Cheol Jo, Yong Park, Ho Sup Lee, Hyo-Jin Kim, Cheolwon Suh
Blood Res. 2017;52(3):200-206. doi: 10.5045/br.2017.52.3.200.Prognostic Significance of Retroperitoneal Lymphadenectomy, Preoperative Neutrophil Lymphocyte Ratio and Platelet Lymphocyte Ratio in Primary Fallopian Tube Carcinoma: A Multicenter Study
Kemal Gungorduk, Ibrahim E. Ertas, Aykut Ozdemir, Emrah Akkaya, Elcin Telli, Salih Taskin, Mehmet Gokcu, Ahmet Baris Guzel, Tufan Oge, Levent Akman, Tayfun Toptas, Ulas Solmaz, Askın Dogan, Mustafa Cosan Terek, Muzaffer Sanci, Aydin Ozsaran, Tayup Simsek, Mehmet Ali Vardar, Omer Tarik Yalcin, Sinan Ozalp, Yusuf Yildirim, Firat Ortac
Cancer Res Treat. 2015;47(3):480-488. doi: 10.4143/crt.2014.058.Systemic Inflammatory Response Markers and CA-125 Levels in Ovarian Clear Cell Carcinoma: A Two Center Cohort Study
Hee Seung Kim, Hwa-Young Choi, Maria Lee, Dong Hoon Suh, Kidong Kim, Jae Hong No, Hyun Hoon Chung, Yong Beom Kim, Yong Sang Song
Cancer Res Treat. 2016;48(1):250-258. doi: 10.4143/crt.2014.324.
Reference
-
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010. 60:277–300.2. Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007. 25:2873–2883.3. McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996. 334:1–6.4. Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001. 19:1001–1007.5. Fathalla MF. Factors in the causation and incidence of ovarian cancer. Obstet Gynecol Surv. 1972. 27:751–768.6. Kodama S, Tanaka K, Tokunaga A, Sudo N, Takahashi T, Matsui K. Multivariate analysis of prognostic factors in patients with ovarian cancer stage I and II. Int J Gynaecol Obstet. 1997. 56:147–153.7. Vergote IB, Kaern J, Abeler VM, Pettersen EO, De Vos LN, Trope CG. Analysis of prognostic factors in stage I epithelial ovarian carcinoma: importance of degree of differentiation and deoxyribonucleic acid ploidy in predicting relapse. Am J Obstet Gynecol. 1993. 169:40–52.8. den Ouden M, Ubachs JM, Stoot JE, van Wersch JW. Whole blood cell counts and leucocyte differentials in patients with benign or malignant ovarian tumours. Eur J Obstet Gynecol Reprod Biol. 1997. 72:73–77.9. Li AJ, Madden AC, Cass I, Leuchter RS, Lagasse LD, Karlan BY. The prognostic significance of thrombocytosis in epithelial ovarian carcinoma. Gynecol Oncol. 2004. 92:211–214.10. Soonthornthum T, Suraseraneewong V, Kengsakol K, Wijaithum K, Kasemsan P, Prommatt S. Thrombocytosis in advanced epithelial ovarian cancer. J Med Assoc Thai. 2007. 90:1495–1500.11. Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964. 114:497–500.12. Tuszynski GP, Nicosia RF. The role of thrombospondin-1 in tumor progression and angiogenesis. Bioessays. 1996. 18:71–76.13. Thavaramara T, Phaloprakarn C, Tangjitgamol S, Manusirivithaya S. Role of neutrophil to lymphocyte ratio as a prognostic indicator for epithelial ovarian cancer. J Med Assoc Thai. 2011. 94:871–877.14. Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009. 58:15–23.15. Asher V, Lee J, Innamaa A, Bali A. Preoperative platelet lymphocyte ratio as an independent prognostic marker in ovarian cancer. Clin Transl Oncol. 2011. 13:499–503.16. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001. 357:539–545.17. Jackson JR, Seed MP, Kircher CH, Willoughby DA, Winkler JD. The codependence of angiogenesis and chronic inflammation. FASEB J. 1997. 11:457–465.18. McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003. 90:215–219.19. Assoian RK, Sporn MB. Type beta transforming growth factor in human platelets: release during platelet degranulation and action on vascular smooth muscle cells. J Cell Biol. 1986. 102:1217–1223.20. Dubernard V, Arbeille BB, Lemesle MB, Legrand C. Evidence for an alpha-granular pool of the cytoskeletal protein alpha-actinin in human platelets that redistributes with the adhesive glycoprotein thrombospondin-1 during the exocytotic process. Arterioscler Thromb Vasc Biol. 1997. 17:2293–2305.21. Kaplan KL, Broekman MJ, Chernoff A, Lesznik GR, Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979. 53:604–618.22. Qian X, Tuszynski GP. Expression of thrombospondin-1 in cancer: a role in tumor progression. Proc Soc Exp Biol Med. 1996. 212:199–207.23. Dabrow MB, Francesco MR, McBrearty FX, Caradonna S. The effects of platelet-derived growth factor and receptor on normal and neoplastic human ovarian surface epithelium. Gynecol Oncol. 1998. 71:29–37.24. Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012. 366:610–618.25. Wang DS, Ren C, Qiu MZ, Luo HY, Wang ZQ, Zhang DS, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol. 2012. 33:749–756.26. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010. 200:197–203.27. Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008. 12:1422–1428.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Thrombocytosis in Patients with Epithelial Ovarian Cancer
- Expression of p21WAF1/CIP1/SDI1 in Epithelial Ovarian Cancer ; Its Relationship with p53 Expression and Prognostic Factors
- A Significance of Thrombocytosis as a Prognostic Factor in Patient with Epithelial Ovarian Cancer
- Prognostic factors of secondary cytoreductive surgery for patients with recurrent epithelial ovarian cancer
- Prognostic Significance of the Neutrophil-Lymphocyte Ratio and Platelet-Lymphocyte Ratio in Neuroendocrine Carcinoma