J Gynecol Oncol.
2012 Oct;23(4):242-250. 10.3802/jgo.2012.23.4.242.
Pelvic exenteration for recurrent cervical cancer: ten-year experience at National Cancer Center in Korea
- Affiliations
-
- 1Center for Uterine Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. parksang@ncc.re.kr
- KMID: 1810121
- DOI: http://doi.org/10.3802/jgo.2012.23.4.242
Abstract
OBJECTIVE
To evaluate survival and morbidity after pelvic exenteration (PE) for the curative management of recurrent cervical cancer.
METHODS
We retrospectively evaluated patients with recurrent cervical cancer who underwent PE from January 2001 to April 2011. Patients were identified from the registry of our institution. The clinical status and demographic information was obtained by reviewing the medical records.
RESULTS
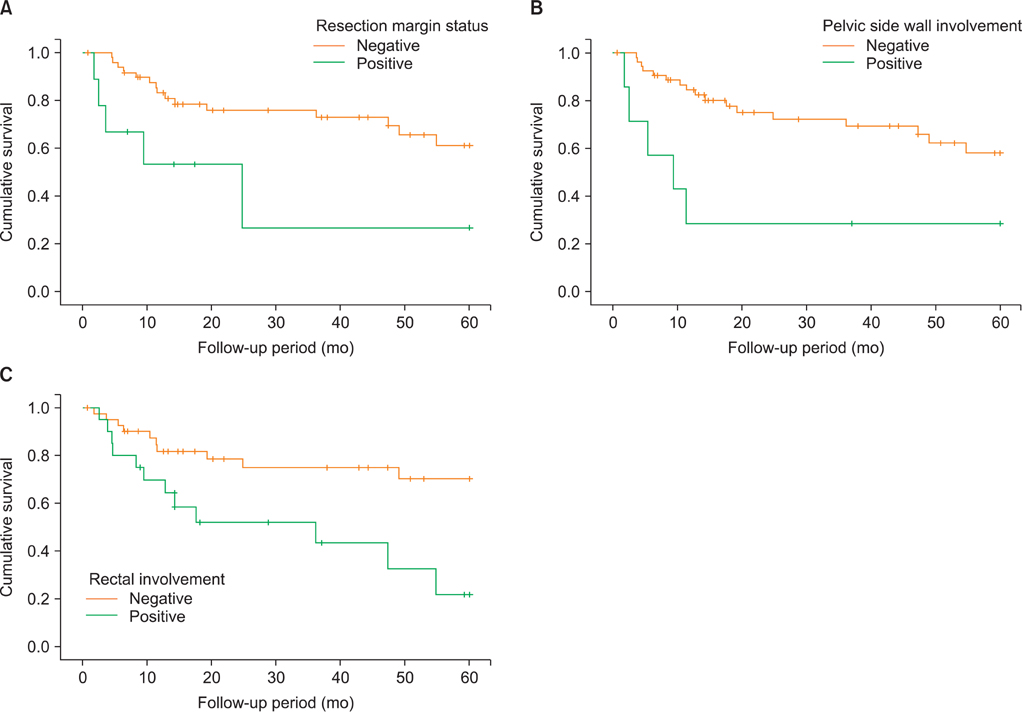
Sixty-one recurrent cervical cancer patients underwent PE. Patients who received radiotherapy, operation, chemotherapy before PE were 98%, 41%, and 23%, respectively. The total morbidity rate was 44%; 10 (16%) patients had early complications (30 days or less after PE), whereas 22 (36%) patients had late complications. Wound problems were common early complications (7/18), and bowel fistulas were common late complications (9/30). The five-year overall survival and five-year disease-free survival were 56% and 49%, respectively. Median follow-up was 22 months (range, 1.8 to 60 months). Affecting factors for overall survival were resection margin status, pelvic wall and rectal involvement.
CONCLUSION
Our overall 5-year survival is encouraging. Although the morbidity rate is still high, PE is a potentially curative opportunity in gynecological malignancies with no other treatment options. The most important factors for overall survival after PE are the resection margin status, pelvic wall involvement and rectal involvement.
MeSH Terms
Figure
Cited by 1 articles
-
Impact of Chemoradiation on Prognosis in Stage IVB Cervical Cancer with Distant Lymphatic Metastasis
Hee Seung Kim, Taehun Kim, Eung Seok Lee, Hak Jae Kim, Hyun Hoon Chung, Jae Weon Kim, Yong Sang Song, Noh Hyun Park
Cancer Res Treat. 2013;45(3):193-201. doi: 10.4143/crt.2013.45.3.193.
Reference
-
1. Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma: a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948. 1:177–183.2. Morley GW, Hopkins MP, Lindenauer SM, Roberts JA. Pelvic exenteration, University of Michigan: 100 patients at 5 years. Obstet Gynecol. 1989. 74:934–943.3. Stanhope CR, Webb MJ, Podratz KC. Pelvic exenteration for recurrent cervical cancer. Clin Obstet Gynecol. 1990. 33:897–909.4. Houvenaeghel G, Moutardier V, Karsenty G, Bladou F, Lelong B, Buttarelli M, et al. Major complications of urinary diversion after pelvic exenteration for gynecologic malignancies: a 23-year mono-institutional experience in 124 patients. Gynecol Oncol. 2004. 92:680–683.5. Kraybill WG, Lopez MJ, Bricker EM. Total pelvic exenteration as a therapeutic option in advanced malignant disease of the pelvis. Surg Gynecol Obstet. 1988. 166:259–263.6. Bladou F, Houvenaeghel G, Delpero JR, Guerinel G. Incidence and management of major urinary complications after pelvic exenteration for gynecological malignancies. J Surg Oncol. 1995. 58:91–96.7. Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol. 2005. 99:153–159.8. Roos EJ, Van Eijkeren MA, Boon TA, Heintz AP. Pelvic exenteration as treatment of recurrent or advanced gynecologic and urologic cancer. Int J Gynecol Cancer. 2005. 15:624–629.9. Sharma S, Odunsi K, Driscoll D, Lele S. Pelvic exenterations for gynecological malignancies: twenty-year experience at Roswell Park Cancer Institute. Int J Gynecol Cancer. 2005. 15:475–482.10. Goldberg GL, Sukumvanich P, Einstein MH, Smith HO, Anderson PS, Fields AL. Total pelvic exenteration: the Albert Einstein College of Medicine/Montefiore Medical Center Experience (1987 to 2003). Gynecol Oncol. 2006. 101:261–268.11. Maggioni A, Roviglione G, Landoni F, Zanagnolo V, Peiretti M, Colombo N, et al. Pelvic exenteration: ten-year experience at the European Institute of Oncology in Milan. Gynecol Oncol. 2009. 114:64–68.12. Benn T, Brooks RA, Zhang Q, Powell MA, Thaker PH, Mutch DG, et al. Pelvic exenteration in gynecologic oncology: a single institution study over 20 years. Gynecol Oncol. 2011. 122:14–18.13. Park JY, Choi HJ, Jeong SY, Chung J, Park JK, Park SY. The role of pelvic exenteration and reconstruction for treatment of advanced or recurrent gynecologic malignancies: analysis of risk factors predicting recurrence and survival. J Surg Oncol. 2007. 96:560–568.14. Fleisch MC, Pantke P, Beckmann MW, Schnuerch HG, Ackermann R, Grimm MO, et al. Predictors for long-term survival after interdisciplinary salvage surgery for advanced or recurrent gynecologic cancers. J Surg Oncol. 2007. 95:476–484.15. Einenkel J, Holler B, Hoffmeister A. Sonographic diagnosis and endo-sponge assisted vacuum therapy of anastomotic leakage following posterior pelvic exenteration for ovarian cancer without using a protective stoma. J Gynecol Oncol. 2011. 22:131–134.16. Galante M, Hill EC. Pelvic exenteration: a critical analysis of a ten-year experience with the use of the team approach. Trans Pac Coast Obstet Gynecol Soc. 1970. 38:59–68.17. Evans RP, Clyburn TA, Moucha CS, Prokuski L. Surgical site infection prevention and control: an emerging paradigm. Instr Course Lect. 2011. 60:539–543.18. Salom EM, Penalver MA. Pelvic exenteration and reconstruction. Cancer J. 2003. 9:415–424.19. Momoh AO, Kamat AM, Butler CE. Reconstruction of the pelvic floor with human acellular dermal matrix and omental flap following anterior pelvic exenteration. J Plast Reconstr Aesthet Surg. 2010. 63:2185–2187.20. Anthopoulos AP, Manetta A, Larson JE, Podczaski ES, Bartholomew MJ, Mortel R. Pelvic exenteration: a morbidity and mortality analysis of a seven-year experience. Gynecol Oncol. 1989. 35:219–223.21. Rutledge FN, Smith JP, Wharton JT, O'Quinn AG. Pelvic exenteration: analysis of 296 patients. Am J Obstet Gynecol. 1977. 129:881–892.22. Shingleton HM, Soong SJ, Gelder MS, Hatch KD, Baker VV, Austin JM Jr. Clinical and histopathologic factors predicting recurrence and survival after pelvic exenteration for cancer of the cervix. Obstet Gynecol. 1989. 73:1027–1034.23. Roberts WS, Cavanagh D, Bryson SC, Lyman GH, Hewitt S. Major morbidity after pelvic exenteration: a seven-year experience. Obstet Gynecol. 1987. 69:617–621.24. Mitulescu G, Ungureanu C, Gluck G, Stingu C, Mitulescu D, Stanculeanu D, et al. Total pelvic exenteration in the treatment of advanced pelvic cancer. Chirurgia (Bucur). 2007. 102:143–154.25. Moriya Y, Akasu T, Fujita S, Yamamoto S. Aggressive surgical treatment for patients with T4 rectal cancer. Colorectal Dis. 2003. 5:427–431.26. Yamada K, Ishizawa T, Niwa K, Chuman Y, Akiba S, Aikou T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001. 88:988–993.27. Mourton SM, Chi DS, Sonoda Y, Alektiar KM, Venkatraman ES, Barakat RR, et al. Mesorectal lymph node involvement and prognostic implications at total pelvic exenteration for gynecologic malignancies. Gynecol Oncol. 2006. 100:533–536.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Total Pelvic Exenteration for Recurrent Cervical Carcinoma Found after Simple Hysterectomy
- Pelvic Exenteration in Treatment of Pelvic Malignancy : 3 Years Experience
- Surgical Management of Recurrent Cervical Cancer
- Diagnosis and Treatment of Recurrent Cervical Cancer
- Pelvic Exenteration as the Treatment for Recurrent or Locally Advanced Rectal Cancer