Allergy Asthma Immunol Res.
2011 Jul;3(3):178-185. 10.4168/aair.2011.3.3.178.
Personal Factors Affecting Therapeutic Responses to BCG Vaccination in Asthmatics
- Affiliations
-
- 1Division of Allergy, Chonnam National University Hospital, Gwangju, Korea. ischoi@chonnam.ac.kr
- 2Division of Allergy and Respiratory Diseases, Soonchunhyang University, Bucheon Hospital, Bucheon, Korea.
- KMID: 1803323
- DOI: http://doi.org/10.4168/aair.2011.3.3.178
Abstract
- PURPOSE
Bacille Calmette-Guerin (BCG) vaccination has been reported to be an effective treatment for asthma in several animal models. This study investigated whether the response to BCG treatment in asthma depends on subject clinical characteristics.
METHODS
Stable asthma patients were vaccinated with BCG. One month later, alterations in pulmonary function after vaccination and their relationships with subject clinical characteristics were determined.
RESULTS
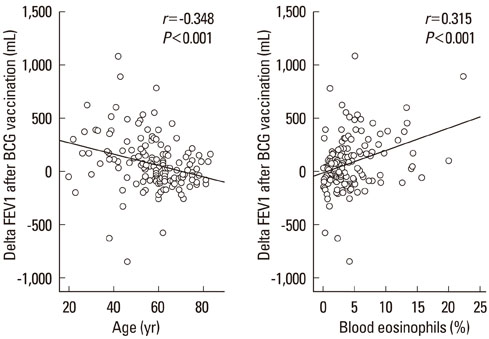
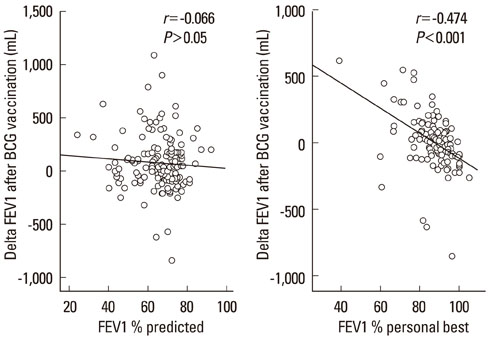
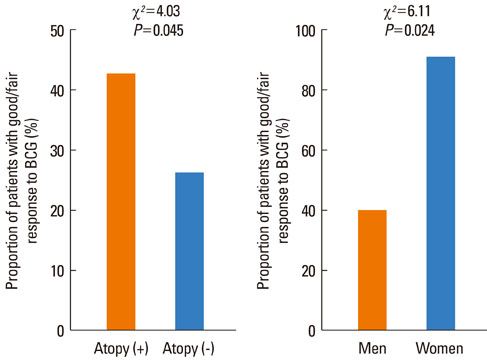
Of 149 patients with asthma, 54 (36.2%) showed a good or fair response to BCG. The DeltaFEV1 after vaccination was significantly related to age (r=-0.348, P<0.001), peripheral blood eosinophil counts (r=0.315, P<0.001) and baseline FEV1, expressed as % personal best value (r=-0.474, P<0.001), but not to FEV1 % predicted value (r=-0.066, P>0.05). A good/fair response was highly prevalent in atopic females compared with atopic males, especially among those aged < or =50 years (90.9% vs. 40.0%, P=0.024). Age (P<0.001, odds ratios (OR)=0.92, confidence interval (CI)=0.88-0.96) and atopy (P<0.01, OR=4.95, CI=1.70-14.44) were significant predictors for a good/fair response in females. However, blood eosinophil counts (P<0.05, OR=1.18, CI=1.01-1.39) and FEV1 % best (P<0.001, OR=0.86, CI=0.79-0.94), but not age or atopy, were significant predictors in males. Approximately three-quarters of the males were smokers.
CONCLUSIONS
The therapeutic effect of BCG in asthma may differ according to patient clinical characteristics. The greatest benefit occurred in young atopic females. Asthma activity indices, such as eosinophilia and FEV1 % best, were more predictive of a good/fair response in males; this may have been related to cigarette smoking.
MeSH Terms
Figure
Reference
-
1. Ownby DR, Johnson CC. Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, editors. Factors underlying the increasing incidence and prevalence of allergic diseases. Middleton's allergy: principles & practice. 2009. 7th ed. Philadelphia: Mosby;769–778.2. Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guérin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998. 187:561–569.3. Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.4. Koh YI, Choi IS, Park SC, Kang KW. BCG infection during pre-sensitization or even post-sensitization inhibits airway sensitivity in an animal model of allergic asthma. J Korean Med Sci. 2000. 15:265–272.5. Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002. 8:625–629.6. Han ER, Choi IS, Eom SH, Kim HJ. Preventive effects of mycobacteria and their culture supernatants against asthma development in BALB/c mice. Allergy Asthma Immunol Res. 2010. 2:34–40.7. Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002. 88:584–591.8. Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guérin in adult asthma. Clin Exp Allergy. 2004. 34:207–212.9. Major T, Wohlleben G, Reibetanz B, Erb KJ. Application of heat killed Mycobacterium bovis-BCG into the lung inhibits the development of allergen-induced Th2 responses. Vaccine. 2002. 20:1532–1540.10. Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005. 95:571–578.11. Choi IS, Lin XH, Koh YA, Cui Y. BCG-induced dendritic cell responses and suppression of interleukin-5 production from T cells in atopic asthmatics. J Korean Med Sci. 2008. 23:628–634.12. Cui Y, Choi IS, Koh YA, Lin XH, Cho YB, Won YH. Effects of combined BCG and DHEA treatment in preventing the development of asthma. Immunol Invest. 2008. 37:191–202.13. Lazarus SC, Chinchilli VM, Rollings NJ, Boushey HA, Cherniack R, Craig TJ, Deykin A, DiMango E, Fish JE, Ford JG, Israel E, Kiley J, Kraft M, Lemanske RF Jr, Leone FT, Martin RJ, Pesola GR, Peters SP, Sorkness CA, Szefler SJ, Wechsler ME, Fahy JV. National Heart Lung and Blood Institute's Asthma Clinical Research Network. Smoking affects response to inhaled corticosteroids or leukotriene receptor antagonists in asthma. Am J Respir Crit Care Med. 2007. 175:783–790.14. Global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma. updated 2009. Available from: http://www.ginasthma.org.15. Cockcroft DW. Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ, editors. Airway responsiveness. Asthma. 1997. Philadelphia: Lippincott-Raven;1253–1266.16. Choi IS, Koh YI. Effects of BCG revaccination on asthma. Allergy. 2003. 58:1114–1116.17. British guidelines on the management of asthma. A national clinical guideline [Internet]. British Thoracic Society Scottish Intercollegiate Guidelines Network. updated 2007 Jul. Available from: http://www.brit-thoracic.org.uk/clinical-information/asthma.18. Morris AH, Kanner RE, Crapo RO, Gardner RM. Clinical pulmonary function testing: a manual of uniform laboratory procedures. 1984. 2nd ed. Salt Lake City: Intermountain Thoracic Society.19. Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis. 1981. 123:659–664.20. Weller PF. Adkinson NF, Bochner BS, Busse WW, Holgate ST, Lemanske RF, Simons FE, editors. Eosinophilia and eosinophil-related disorders. Middleton's allergy: principles & practice. 2009. 7th ed. Philadelphia: Mosby;859–877.21. Miller RA. The aging immune system: primer and prospectus. Science. 1996. 273:70–74.22. Scarpace PJ, Abrass IB. Decreased beta-adrenergic agonist affinity and adenylate cyclase activity in senescent rat lung. J Gerontol. 1983. 38:143–147.23. Armanini D, Scali M, Vittadello G, Ribecco M, Zampollo V, Pratesi C, Orlandini E, Zovato S, Zennaro CM, Karbowiak I. Corticosteroid receptors and aging. J Steroid Biochem Mol Biol. 1993. 45:191–194.24. Choi IS, Lin XH, Koh YA, Cui Y. Inoculation route-dependent and allergen-specific suppressive effects of bacille Calmette-Guérin vaccination on asthmatic reactions in BALB/c mice. Lung. 2007. 185:179–186.25. Choi IS. Gender-specific asthma treatment. Allergy Asthma Immunol Res. 2011. 3:74–80.26. Magnusson CG. Maternal smoking influences cord serum IgE and IgD levels and increases the risk for subsequent infant allergy. J Allergy Clin Immunol. 1986. 78:898–904.27. Adisesh A, Gruszka L, Robinson E, Evans G. Smoking status and immunoglobulin E seropositivity to workplace allergens. Occup Med (Lond). 2011. 61:62–64.28. de Benedictis FM, Baraldi E, Boner A. Gender differences in the effectiveness of asthma treatment. Pediatrics. 2008. 121:1289.29. Choi IS, Cui Y, Koh YA, Lee HC, Cho YB, Won YH. Effects of dehydroepiandrosterone on Th2 cytokine production in peripheral blood mononuclear cells from asthmatics. Korean J Intern Med. 2008. 23:176–181.30. Everitt BS. The Cambridge dictionary of statistics. 2006. 3rd ed. Cambridge: Cambridge University Press.31. Legendre AM, Easley JR, Becker PU. In vivo and in vitro responses of cats sensitized with viable Mycobacterium bovis (BCG). Am J Vet Res. 1979. 40:1613–1619.32. Adinoff AD, Rosloniec DM, McCall LL, Nelson HS. Immediate skin test reactivity to Food and Drug Administration-approved standardized extracts. J Allergy Clin Immunol. 1990. 86:766–774.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- BCG Immunomodulation Therapy in Asthma
- BCG-Induced Dendritic Cell Responses and Suppression of Interleukin-5 Production from T Cells in Atopic Asthmatics
- A Case of Lichen Scrofulosorum Developing after BCG Vaccination
- BCG Osteomyelitis: A Case Report
- Tuberculous Osteomyelitis on the Proximal Humerus after BCG vaccination: a case report