Korean J Radiol.
2014 Dec;15(6):810-816. 10.3348/kjr.2014.15.6.810.
Is Diffusion-Weighted MRI Useful for Differentiation of Small Non-Necrotic Cervical Lymph Nodes in Patients with Head and Neck Malignancies?
- Affiliations
-
- 1Department of Radiology and Research Institute of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea. jeonghlee@amc.seoul.kr
- 2Department of Radiology, Soonchunhyang University Hospital, Seoul 140-743, Korea.
- 3Department of Otolaryngology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- 4Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 138-736, Korea.
- KMID: 1794654
- DOI: http://doi.org/10.3348/kjr.2014.15.6.810
Abstract
OBJECTIVE
To evaluate the usefulness of measuring the apparent diffusion coefficient (ADC) in diffusion-weighted magnetic resonance imaging to distinguish benign from small, non-necrotic metastatic cervical lymph nodes in patients with head and neck cancers.
MATERIALS AND METHODS
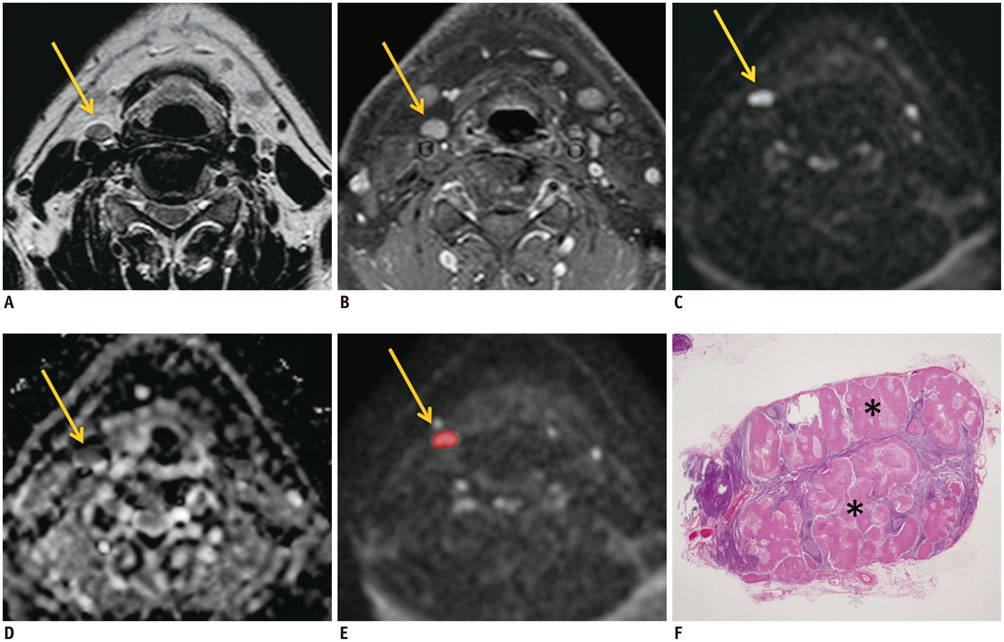
Twenty-six consecutive patients with head and neck cancer underwent diffusion-weighted imaging (b value, 0 and 800 s/mm2) preoperatively between January 2009 and December 2010. Two readers independently measured the ADC values of each cervical lymph node with a minimum-axial diameter of > or = 5 mm but < 11 mm using manually drawn regions of interest. Necrotic lymph nodes were excluded. Mean ADC values were compared between benign and metastatic lymph nodes after correlating the pathology.
RESULTS
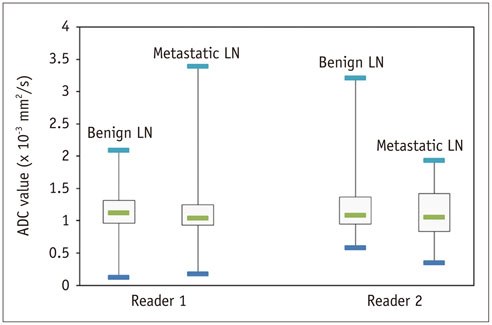
A total of 116 lymph nodes (91 benign and 25 metastatic) from 25 patients were included. Metastatic lymph nodes (mean +/- standard deviation [SD], 7.4 +/- 1.6 mm) were larger than benign lymph nodes (mean +/- SD, 6.6 +/- 1.4 mm) (p = 0.018). Mean ADC values for reader 1 were 1.17 +/- 0.31 x 10-3 mm2/s for benign and 1.25 +/- 0.76 x 10-3 mm2/s for metastatic lymph nodes. Mean ADC values for reader 2 were 1.21 +/- 0.46 x 10-3 mm2/s for benign and 1.14 +/- 0.34 x 10-3 mm2/s for metastatic lymph nodes. Mean ADC values between benign and metastatic lymph nodes were not significantly different (p = 0.594 for reader 1, 0.463 for reader 2).
CONCLUSION
Measuring mean ADC does not allow differentiating benign from metastatic cervical lymph nodes in patients with head and neck cancer and non-necrotic, small lymph nodes.
MeSH Terms
Figure
Cited by 1 articles
-
Diffusion-Weighted MR Imaging of Unicystic Odontogenic Tumors for Differentiation of Unicystic Ameloblastomas from Keratocystic Odontogenic Tumors
Yifeng Han, Xindong Fan, Lixin Su, Zhenfeng Wang
Korean J Radiol. 2018;19(1):79-84. doi: 10.3348/kjr.2018.19.1.79.
Reference
-
1. van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990; 177:379–384.2. van den Brekel MW, Castelijns JA, Snow GB. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: how reliable is it? AJNR Am J Neuroradiol. 1998; 19:695–700.3. Yuasa K, Kawazu T, Nagata T, Kanda S, Ohishi M, Shirasuna K. Computed tomography and ultrasonography of metastatic cervical lymph nodes in oral squamous cell carcinoma. Dentomaxillofac Radiol. 2000; 29:238–244.4. King AD, Tse GM, Ahuja AT, Yuen EH, Vlantis AC, To EW, et al. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology. 2004; 230:720–726.5. Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol. 1992; 158:961–969.6. van den Brekel MW. Lymph node metastases: CT and MRI. Eur J Radiol. 2000; 33:230–238.7. Ng SH, Yen TC, Chang JT, Chan SC, Ko SF, Wang HM, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography and computed tomography and magnetic resonance imaging in oral cavity squamous cell carcinoma with palpably negative neck. J Clin Oncol. 2006; 24:4371–4376.8. Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med. 2007; 48:726–735.9. Vandecaveye V, De Keyzer F, Vander Poorten V, Dirix P, Verbeken E, Nuyts S, et al. Head and neck squamous cell carcinoma: value of diffusion-weighted MR imaging for nodal staging. Radiology. 2009; 251:134–146.10. Sumi M, Sakihama N, Sumi T, Morikawa M, Uetani M, Kabasawa H, et al. Discrimination of metastatic cervical lymph nodes with diffusion-weighted MR imaging in patients with head and neck cancer. AJNR Am J Neuroradiol. 2003; 24:1627–1634.11. Abdel Razek AA, Soliman NY, Elkhamary S, Alsharaway MK, Tawfik A. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radiol. 2006; 16:1468–1477.12. de Bondt RB, Hoeberigs MC, Nelemans PJ, Deserno WM, Peutz-Kootstra C, Kremer B, et al. Diagnostic accuracy and additional value of diffusion-weighted imaging for discrimination of malignant cervical lymph nodes in head and neck squamous cell carcinoma. Neuroradiology. 2009; 51:183–192.13. Holzapfel K, Duetsch S, Fauser C, Eiber M, Rummeny EJ, Gaa J. Value of diffusion-weighted MR imaging in the differentiation between benign and malignant cervical lymph nodes. Eur J Radiol. 2009; 72:381–387.14. Perrone A, Guerrisi P, Izzo L, D'Angeli I, Sassi S, Mele LL, et al. Diffusion-weighted MRI in cervical lymph nodes: differentiation between benign and malignant lesions. Eur J Radiol. 2011; 77:281–286.15. Jansen JF, Stambuk HE, Koutcher JA, Shukla-Dave A. Non-gaussian analysis of diffusion-weighted MR imaging in head and neck squamous cell carcinoma: a feasibility study. AJNR Am J Neuroradiol. 2010; 31:741–748.16. King AD, Ahuja AT, Yeung DK, Fong DK, Lee YY, Lei KI, et al. Malignant cervical lymphadenopathy: diagnostic accuracy of diffusion-weighted MR imaging. Radiology. 2007; 245:806–813.17. Wu LM, Xu JR, Liu MJ, Zhang XF, Hua J, Zheng J, et al. Value of magnetic resonance imaging for nodal staging in patients with head and neck squamous cell carcinoma: a meta-analysis. Acad Radiol. 2012; 19:331–340.18. Gallus J, Mathiowetz V. Test-retest reliability of the Purdue Pegboard for persons with multiple sclerosis. Am J Occup Ther. 2003; 57:108–111.19. Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007; 188:1622–1635.20. Lee SC, Moon WJ, Choi JW, Roh HG, Bak SH, Yi JG, et al. Differentitation between primary central nervous system lymphoma and glioblastoma: added value of quantitative analysis of CT attenuation and apparent diffusion coefficient. J Korean Soc Magn Reson Med. 2012; 16:226–235.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CT and MR Findings of Kimura's Disease in the Head and Neck
- A case report of castleman disease in th neck: CT and MRI finding
- The evaluation of the cervical lymph node metastases using computed tomography in the head and neck cancer
- The Value of PROPELLER Diffusion-Weighted Image in the Detection of Cholesteatoma
- Ultrasonographic Evaluation of Cervical Lymph Nodes