Yonsei Med J.
2008 Aug;49(4):639-646. 10.3349/ymj.2008.49.4.639.
A Multi-institutional Study on Histopathological Characteristics of Surgically Treated Renal Tumors: the Importance of Tumor Size
- Affiliations
-
- 1Department of Urology, Ajou University School of Medicine, Suwon, Korea. sjhong346@yuhs.ac
- 2Department of Urology, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Urology, Inha University College of Medicine, Incheon, Korea.
- 4Department of Urology, Keimyung University School of Medicine, Daegu, Korea.
- 5Department of Urology, University of Ulsan College of Medicine, Ulsan, Korea.
- 6Department of Urology, Hallym University College of Medicine, Anyang, Korea.
- 7Department of Urology, Soonchunhyang University College of Medicine, Seoul, Korea.
- 8Department of Urology, National Institute of Health Corporation Ilsan Hospital, Goyang, Korea.
- 9Department of Urology, Inje University College of Medicine, Goyang, Korea.
- 10Department of Urology, Ewha Woman's University College of Medicine, Seoul, Korea.
- 11Department of Urology, Konyang University College of Medicine, Daejeon, Korea.
- 12Department of Urology, Konkuk University School of Medicine, Chungju, Korea.
- 13Department of Urology, Sungkyunkwan University College of Medicine, Seoul, Korea.
- KMID: 1793201
- DOI: http://doi.org/10.3349/ymj.2008.49.4.639
Abstract
- PURPOSE
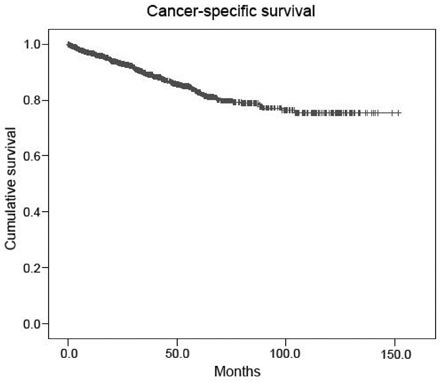
The incidence of accidentally detected small renal tumors is increasing throughout the world. In this multi-institutional study performed in Korea, histopathological characteristics of contemporarily surgically removed renal tumors were reviewed with emphasis on tumor size. MATERIALS and METHODS: Between January 1995 and May 2005, 1,702 patients with a mean age of 55 years underwent surgical treatment at 14 training hospitals in Korea for radiologically suspected malignant renal tumors. Clinicopathological factors and patient survival were analyzed. RESULTS: Of the 1,702 tumors, 91.7% were malignant and 8.3% were benign. The percentage of benign tumors was significantly greater among those < or = 4cm (13.2%) than those > 4cm (4.5%) (p < 0.001). Among renal cell carcinoma patients, the percentage of tumors classed as stage > or = T3 was significantly less among tumors < or = 4cm (5.2%) than those > 4cm (26.8%) (p < 0.001). The percentage of tumors classed as Fuhrman's nuclear grades > or = 3 was also significantly less among tumors < or = 4cm (27.3%) than tumors > 4cm (50.9%) (p < 0.001). The 5-year cancer-specific survival rate was 82.7%, and T stage (p < 0.001), N stage (p < 0.001), M stage (p = 0.025), and Fuhrman's nuclear (p < 0.001) grade were the only independent predictors of cancer-specific survival. CONCLUSION: In renal tumors, small tumor size is prognostic for favorable postsurgical histopathologies such as benign tumors, low T stages, and low Fuhrman's nuclear grades. Our observations are expected to facilitate urologists to adopt function-preserving approach in the planning of surgery for small renal tumors with favorable predicted outcomes.
MeSH Terms
Figure
Cited by 1 articles
-
Trends of Presentation and Clinical Outcome of Treated Renal Angiomyolipoma
Kyo Chul Koo, Won Tae Kim, Won Sik Ham, Jin Sun Lee, Hee Jeong Ju, Young Deuk Choi
Yonsei Med J. 2010;51(5):728-734. doi: 10.3349/ymj.2010.51.5.728.
Reference
-
1. Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998. 51:203–205.
Article2. Patard JJ, Tazi H, Bensalah K, Rodriguez A, Vincendeau S, Rioux-Leclercq N, et al. The changing evolution of renal tumours: a single center experience over a two-decade period. Eur Urol. 2004. 45:490–494.
Article3. Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology. 2000. 56:58–62.4. Duchene DA, Lotan Y, Cadeddu JA, Sagalowsky AI, Koeneman KS. Histopathology of surgically managed renal tumors: analysis of a contemporary series. Urology. 2003. 62:827–830.
Article5. Lee CT, Katz J, Shi W, Thaler HT, Reuter VE, Russo P. Surgical management of renal tumors 4 cm. or less in a contemporary cohort. J Urol. 2000. 163:730–736.
Article6. Lam JS, Shvarts O, Pantuck AJ. Changing concepts in the surgical management of renal cell carcinoma. Eur Urol. 2004. 45:692–705.
Article7. Marszalek M, Ponholzer A, Brössner C, Wachter J, Maier U, Madersbacher S. Elective open nephron-sparing surgery for renal masses: single-center experience with 129 consecutive patients. Urology. 2004. 64:38–42.
Article8. Dechet CB, Sebo T, Farrow G, Blute ML, Engen DE, Zincke H. Prospective analysis of intraoperative frozen needle biopsy of solid renal masses in adults. J Urol. 1999. 162:1282–1284. discussion 1284-5.
Article9. Guinan P, Sobin LH, Algaba F, Badellino F, Kameyama S, MacLennan G, et al. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer. 1997. 80:992–993.10. Skinner DG, Colvin RB, Vermillion CD, Pfister RC, Leadbetter WF. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971. 28:1165–1177.
Article11. Valeri A, Lang H, Taccoen X, Skowron O, Descotes JL, Coulange C, et al. Pathological features of kidney masses 4 cm or less: the less frequently malignant nature compared to larger tumours, supports the interest of nephron sparing surgery (abstract). Eur Urol Suppl. 2005. 4:49.12. Lieber MM. Renal oncocytoma. Urol Clin North Am. 1993. 20:355–359.
Article13. Yen TH, Chen Y, Lin JL, Ng KF. Renal oncocytoma in Taiwan. Ren Fail. 2006. 28:141–147.
Article14. Jeschke K, Peschel R, Wakonig J, Schellander L, Bartsch G, Henning K. Laparoscopic nephron-sparing surgery for renal tumors. Urology. 2001. 58:688–692.
Article15. Snyder ME, Bach A, Kattan MW, Raj GV, Reuter VE, Russo P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. J Urol. 2006. 176:2391–2395. discussion 2395-6.
Article16. Lemaitre L, Claudon M, Dubrulle F, Mazeman E. Imaging of angiomyolipomas. Semin Ultrasound CT MR. 1997. 18:100–114.
Article17. Milner J, McNeil B, Alioto J, Proud K, Rubinas T, Picken M, et al. Fat poor renal angiomyolipoma: patient, computerized tomography and histological findings. J Urol. 2006. 176:905–909.
Article18. Dechet CB, Zincke H, Sebo TJ, King BF, LeRoy AJ, Farrow GM, et al. Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol. 2003. 169:71–74.
Article19. Rybicki FJ, Shu KM, Cibas ES, Fielding JR, vanSonnenberg E, Silverman SG. Percutaneous biopsy of renal masses: sensitivity and negative predictive value stratified by clinical setting and size of masses. AJR Am J Roentgenol. 2003. 180:1281–1287.
Article20. Kim H, Cho NH, Kim DS, Kwon YM, Kim EK, Rha SH, et al. Genitourinary Pathology Study Group of the Korean Society of Pathologists. Renal cell carcinoma in South Korea: a multicenter study. Hum Pathol. 2004. 35:1556–1563.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Analysis of Radiologically Measured Size and True Size of Renal Tumors
- Clinical Observations on 63 Cases of Renal Tumors
- Correlation of CT Findings and Pathologic Nuclear Grading in Renal Cell Carcinoma
- Radiofrequency Ablation of Unilateral Kidney VX2 Tumors in the Rabbit Model
- The Characteristics of Renal Trauma Patients Who Needed Surgical Management