J Korean Med Assoc.
2012 Sep;55(9):819-826. 10.5124/jkma.2012.55.9.819.
Recent international initiatives of drug safety management
- Affiliations
-
- 1Medical Research Collaborating Center, Seoul National University Hospital/Seoul National University College of Medicine, Seoul, Korea. bjpark@snu.ac.kr
- 2Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Korea Institute of Drug Safety and Risk Management, Seoul, Korea.
- KMID: 1787244
- DOI: http://doi.org/10.5124/jkma.2012.55.9.819
Abstract
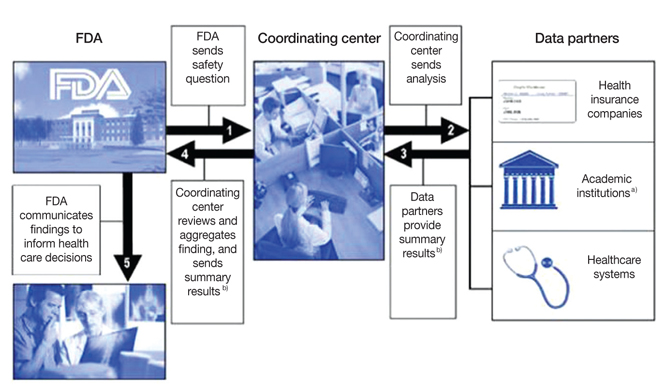
- Adverse drug reactions constitute a major public health problem. In recent years, serious safety issues arose with marketed drugs, and public outcry demanded better safety surveillance. Now regulatory focus is shifting to the active post-marketing safety surveillance. This paper provides an overview of the recent international initiatives of drug safety management especially focused on the US and Europe. The US Food and Drug Administration's (FDA) Sentinel Initiative is a long-term program designed to build and implement a national electronic system for monitoring the safety of FDA-approved drugs and other medical products. The Sentinel System will enable FDA to monitor the safety of medical products with the assistance of a wide array of collaborating institutions throughout the nation. The European Network of Centers for Pharmacoepidemiology and Pharmacovigilance is a collaborative scientific network coordinated by the European Medicines Agency and developed in collaboration with European experts in the fields of pharmacoepidemiology and pharmacovigilance. Its goal is to further strengthen the post-marketing monitoring of medicinal products in Europe by facilitating the conduct of multi-center, independent, post-authorization studies focusing on safety and on benefit-risk. Medicine is a global enterprise that demands worldwide standards for good drug safety practices. In the near future, we have to establish a Korean Sentinel System for active post-marketing safety surveillance to ensure the safety and effectiveness of drugs used in medical practice.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Application of big data for public health
Byung-Joo Park
J Korean Med Assoc. 2014;57(5):383-385. doi: 10.5124/jkma.2014.57.5.383.Use of big data for drug safety monitoring and decision making
Sun-Young Jung, Nam-Kyong Choi, Joongyub Lee, Byung-Joo Park
J Korean Med Assoc. 2014;57(5):391-397. doi: 10.5124/jkma.2014.57.5.391.Past, present, and future of pharmacovigilance in Korea
Dong Yoon Kang, Kyung-Min Ahn, Hye-Ryun Kang, Sang-Heon Cho
Asia Pac Allergy. 2017;7(3):173-178. doi: 10.5415/apallergy.2017.7.3.173.
Reference
-
1. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961. 2:1358.
Article2. Pannikar V. The return of thalidomide: new uses and renewed concerns. Lepr Rev. 2003. 74:286–288.3. Waxman HA. The lessons of Vioxx: drug safety and sales. N Engl J Med. 2005. 352:2576–2578.4. Baciu A, Stratton KR, Burke SP. Institute of Medicine (US), Committee on the Assessment of the US Drug Safety System. Institute of Medicine (US), Board on Population Health and Public Health Practice. The future of drug safety: promoting and protecting the health of the public. 2006. Washington, DC: National Academies Press;348.5. Choi NK, Park BJ. Adverse drug reaction surveillance system in Korea. J Prev Med Public Health. 2007. 40:278–284.
Article6. Von Eschenbach AC. The FDA Amendments Act: reauthorization of the FDA. Food Drug Law J. 2008. 63:579–584.7. The Food and Drug Administration Amendments Act of 2007, Pub. L. No. 110-85, 121 Stat. 823 (Sep 27, 2007), amending the Federal Food, Drug, and Cosmetic Act, 21 USC 301.8. US Food and Drug Administration. FDA's Sentinel Initiative [Internet]. cited 2012 Aug 16. Silver Spring: US Food and Drug Administration;Available from: http://www.fda.gov/Safety/FDAsSentinelInitiative/default.htm.9. Behrman RE, Benner JS, Brown JS, McClellan M, Woodcock J, Platt R. Developing the Sentinel System: a national resource for evidence development. N Engl J Med. 2011. 364:498–499.
Article10. Platt R, Carnahan RM, Brown JS, Chrischilles E, Curtis LH, Hennessy S, Nelson JC, Racoosin JA, Robb M, Schneeweiss S, Toh S, Weiner MG. The U.S. Food and Drug Administration's Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012. 21:Suppl 1. 1–8.
Article11. Wood SF, Perosino KL. Increasing transparency at the FDA: the impact of the FDA Amendments Act of 2007. Public Health Rep. 2008. 123:527–530.
Article12. Forrow S, Campion DM, Herrinton LJ, Nair VP, Robb MA, Wilson M, Platt R. The organizational structure and governing principles of the Food and Drug Administration's Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf. 2012. 21:Suppl 1. 12–17.
Article13. US Department of Health and Human Services. US Food and Drug Administration. The Sentinel Initiative: a national strategy for monitoring medical product safety [Internet]. 2011. cited 2012 Sep 7. Silver Spring: US Food and Drug Administration;Available from: http://www.fda.gov/downloads/Safety/FDAsSentinelInitiative/UCM274548.pdf.14. Nguyen M, Ball R, Midthun K, Lieu TA. The Food and Drug Administration's Post-Licensure Rapid Immunization Safety Monitoring program: strengthening the federal vaccine safety enterprise. Pharmacoepidemiol Drug Saf. 2012. 21:Suppl 1. 291–297.
Article15. Fireman B, Toh S, Butler MG, Go AS, Joffe HV, Graham DJ, Nelson JC, Daniel GW, Selby JV. A protocol for active surveil-lance of acute myocardial infarction in association with the use of a new antidiabetic pharmaceutical agent. Pharmacoepidemiol Drug Saf. 2012. 21:Suppl 1. 282–290.
Article16. Permanand G, Mossialos E, McKee M. Regulating medicines in Europe: the European Medicines Agency, marketing authorisation, transparency and pharmacovigilance. Clin Med. 2006. 6:87–90.
Article17. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. What is ENCePP [Internet]. cited 2012 Aug 16. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance;Available from: http://www.encepp.eu/structure/index.html.18. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. ENCePP steering group [Internet]. cited 2012 Aug 16. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance;Available from: http://www.encepp.eu/structure/structure_steeringGroup.html.19. European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. ENCePP studies [Internet]. cited 2012 Aug 16. London: European Network of Centres for Pharmacoepidemiology and Pharmacovigilance;Available from: http://www.encepp.eu/encepp_studies/index.html.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Change of the Etiology of Drug Induced Anaphylaxis in a Korean Tertiary Care Hospital
- Lessons from US FDA's COVID-19 Related Post-Marketing Drug Safety Monitoring and Evaluation Activities
- Canonical Correlation between Drug Dosage Calculation Error Prevention Competence of Nurses and Medication Safety Organizational Climate
- A Study on the Pilot Fatigue Measurement Methods for Fatigue Risk Management
- Comparison of Expedited Reporting System for Investigational Drugs in the U .S., EU and South Korea