J Korean Med Sci.
2008 Feb;23(1):122-125. 10.3346/jkms.2008.23.1.122.
Parent-Controlled Analgesia in Children Undergoing Cleft Palate Repair
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, and Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. ktmin501@yumc.yonsei.ac.kr
- 2Department of Anesthesiology and Pain Medicine, Kwandong University College of Medicine, Goyang, Korea.
- 3Department of Plastic and Reconstructive Surgery, Institute for Human Tissue Restoration, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 1786857
- DOI: http://doi.org/10.3346/jkms.2008.23.1.122
Abstract
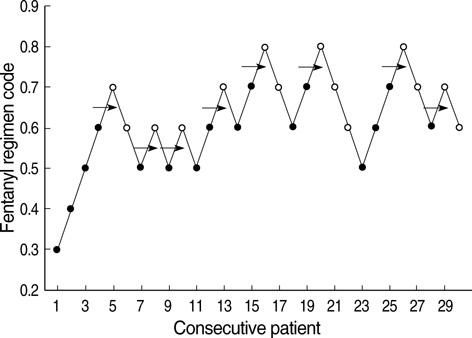
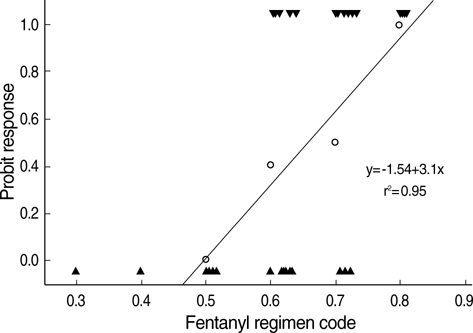
- The aims of this study were to find an optimal basal infusion dose of fentanyl for parent-controlled analgesia (PrCA) in children undergoing cleft palate repair and the degree of parents' satisfaction with PrCA. Thirty consecutive children between 6 months and 2 yr of age were enrolled. At the end of surgery, a PrCA device with a basal infusion rate of 2 mL/hr and bolus of 0.5 mL with lockout time of 15 min was applied. Parents were educated in patient-controlled analgesia (PCA) devices, the Wong Baker face pain scoring system, and monitoring of adverse effects of fentanyl. Fentanyl was infused 0.3 microgram/kg/hr at first, and we obtained a predetermined fentanyl regimen by the response of the previous patient to a larger or smaller dose of fentanyl (0.1 microgram/kg/hr as the step size), using an up-and-down method. ED50 and ED95 by probit analysis were 0.63 microgram/kg/hr (95% confidence limits, 0.55-0.73 microgram/kg/hr) and 0.83 microgram/kg/hr (95% confidence limits, 0.73-1.47 microgram/kg/hr), respectively. Eighty seven percent of the parents were satisfied with participating in the PrCA modality. PrCA using fentanyl with a basal infusion rate of 0.63 microgram/kg/hr can be applied effectively for postoperative pain management in children undergoing cleft palate repair with a high level of parents' satisfaction.
MeSH Terms
Figure
Cited by 1 articles
-
Small dose of propofol combined with dexamethasone for postoperative vomiting in pediatric Moyamoya disease patients: a prospective, observer-blinded, randomized controlled study
Jeongmin Kim, Gyu Dong Jang, Dong-Suk Kim, Kyeong Tae Min
Korean J Anesthesiol. 2013;64(2):127-132. doi: 10.4097/kjae.2013.64.2.127.
Reference
-
1. Munro HM, Malviya S, Lauder GR, Voepel-Lewis T, Tait AR. Pain relief in children following outpatient surgery. J Clin Anesth. 1999. 11:187–191.
Article2. Peters JW, Bandell Hoekstra IE, Huijer Abu-Saad H, Bouwmeester J, Meursing AE, Tibboel D. Patient controlled analgesia in children and adolescents: a randomized controlled trial. Paediatr Anaesth. 1999. 9:235–241.
Article3. Verghese ST, Hannallah RS. Postoperative pain management in children. Anesthesiol Clin North America. 2005. 23:163–184.
Article4. Kanagasundaram SA, Cooper MG, Lane LJ. Nurse-controlled analgesia using a patient-controlled analgesia device: an alternative strategy in the management of severe cancer pain in children. J Paediatr Child Health. 1997. 33:352–355.
Article5. Roulleau P, Gall O, Desjeux L, Dagher C, Murat I. Remifentanil infusion for cleft palate surgery in young infants. Paediatr Anaesth. 2003. 13:701–707.
Article6. Kehlet H. Procedure-specific postoperative pain management. Anesthesiol Clin North America. 2005. 23:203–210.
Article7. Dell'Oste C, Savron F, Pelizzo G, Sarti A. Acute airway obstruction in an infant with Pierre Robin syndrome after palatoplasty. Acta Anaesthesiol Scand. 2004. 48:787–789.8. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991. 15:47–50.
Article9. Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988. 14:9–17.10. Klimscha W, Chiari A, Michalek-Sauberer A, Wildling E, Lerche A, Lorber C, Brinkmann H, Semsroth M. The efficacy and safety of a clonidine/bupivacaine combination in caudal blockade for pediatric hernia repair. Anesth Analg. 1998. 86:54–61.
Article11. Buttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on seven consecutive studies. Paediatr Anaesth. 2000. 10:303–318.12. Monitto CL, Greenberg RS, Kost-Byerly S, Wetzel R, Billett C, Lebet RM, Yaster M. The safety and efficacy of parent-/nurse-controlled analgesia in patients less than six years of age. Anesth Analg. 2000. 91:573–579.
Article13. Katz R, Kelly HW. Pharmacokinetics of continuous infusions of fentanyl in critically ill children. Crit Care Med. 1993. 21:995–1000.
Article14. Yildiz K, Tercan E, Dogru K, Ozkan U, Boyaci A. Comparison of patient-controlled analgesia with and without a background infusion after appendicectomy in children. Paediatr Anaesth. 2003. 13:427–431.
Article15. Kelly AM, Powell CV, Williams A. Parent visual analogue scale ratings of children's pain do not reliably reflect pain reported by child. Pediatr Emerg Care. 2002. 18:159–162.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative study of levobupivacaine and bupivacaine for bilateral maxillary nerve block during pediatric primary cleft palate surgery: a randomized double-blind controlled study
- Dexmedetomidine during suprazygomatic maxillary nerve block for pediatric cleft palate repair, randomized double-blind controlled study
- A novel modification of Bardach’s two-flap palatoplasty for the repair of a difficult cleft palate
- Cause analysis, prevention, and treatment of postoperative restlessness after general anesthesia in children with cleft palate
- Clinical Experience of Cleft Lip and/or Palate Repair in Complex Congenital Heart Disease