Korean J Radiol.
2005 Mar;6(1):8-16. 10.3348/kjr.2005.6.1.8.
Assessment of the Prognostic Factors for a Local Recurrence of Rectal Cancer: the Utility of Preoperative MR Imaging
- Affiliations
-
- 1Department of Diagnostic Radiology, Yonsei University College of Medicine, Korea. kimnex@yumc.yonsei.ac.kr
- 2Department of Surgery, Yonsei University College of Medicine, Korea.
- 3Department of Internal Medicine, Gastroenterology Division, Yonsei University College of Medicine, Korea.
- 4Brain Korea 21 Project of Medical Science, Yonsei University College of Medicine, Korea.
- KMID: 1783167
- DOI: http://doi.org/10.3348/kjr.2005.6.1.8
Abstract
OBJECTIVE
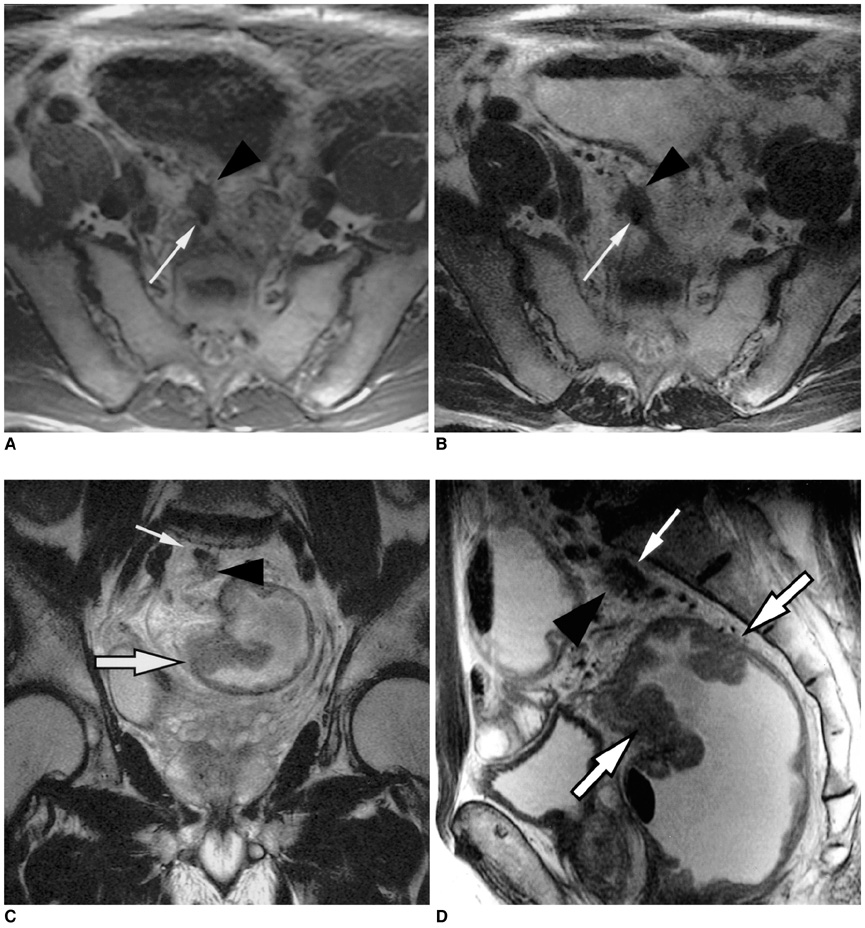
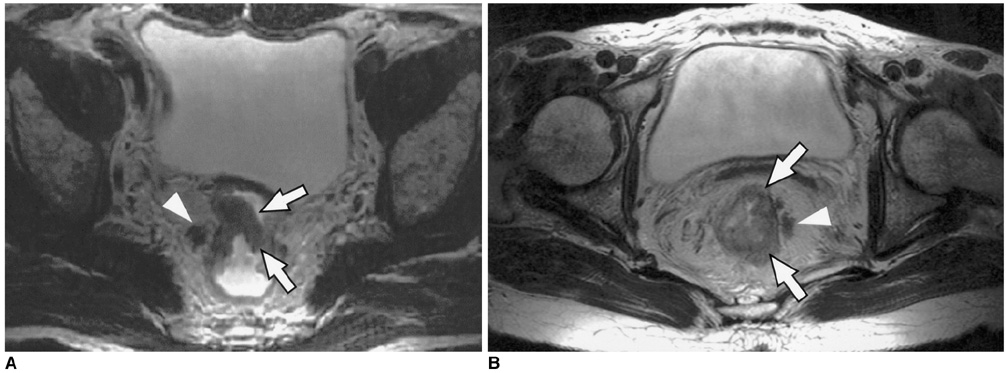
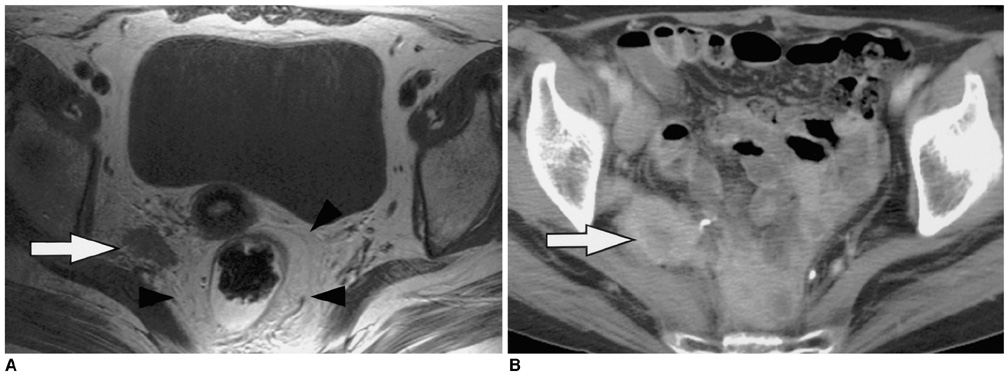
To determine the utility of MR imaging in evaluating the prognostic factors for a local recurrence of rectal cancer following a curative resection. MATERIALS AND METHODS: The preoperative MR images obtained from 17 patients with a local recurrence and 54 patients without a local recurrence, who had undergone a curative resection, were independently evaluated by three radiologists. The following findings were analyzed: the direct invasion of the perirectal fat by the primary rectal carcinoma, involvement of the perirectal lymph nodes, perirectal spiculate nodules, perivascular encasement, and an enlargement of the pelvic wall lymph nodes. The clinical and surgical profiles were obtained from the patients' medical records. The association of a local recurrence with the MR findings and the clinicosurgical variables was statistically evaluated. RESULTS: Of the MR findings, the presence of perivascular encasement (p = 0.001) and perirectal spiculate nodules (p = 0.001) were found to be significant prognostic factors for a local recurrence. Of the clinicosurgical profiles, the presence of a microscopic vascular invasion (p = 0.005) and the involvement of the regional lymph nodes (p = 0.006) were associated with a local recurrence. Logistic regression analysis showed that the presence of perirectal spiculate nodules was an independent predictor of a local recurrence (odds ratio, 7.382; 95% confidence interval, 1.438, 37.889; p = 0.017). CONCLUSION: The presence of perirectal spiculate nodules and perivascular encasement on the preoperative MR images are significant predictors of a local recurrence after curative surgery for a rectal carcinoma. This suggests that preoperative MR imaging can provide useful information to help in the planning of preoperative adjuvant therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Ross A, Rusnak C, Weinerman B, Kuechler P, Hayashi A, Maclachlan G, et al. Recurrence and survival after surgical management of rectal cancer. Am J Surg. 1999. 177:392–395.2. Tveit KM, Guldvog I, Hagen S, Trondsen E, Harbitz T, Nygaard K, et al. Norwegian Adjuvant Rectal Cancer Project Group. Randomized controlled trial of postoperative radiotherapy and short-term time-scheduled 5-fluorouracil against surgery alone in the treatment of Dukes B and C rectal cancer. Br J Surg. 1997. 84:1130–1135.3. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986. 1:1479–1482.4. Reynolds JV, Joyce WP, Dolan J, Sheahan K, Hyland JM. Pathological evidence in support of total mesorectal excision in the management of rectal cancer. Br J Surg. 1996. 83:1112–1115.5. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001. 345:638–646.6. Chapuis PH, Dent OF, Fisher R, Newland RC, Pheils MT, Smyth E, et al. A multivariate analysis of clinical and pathological variables in prognosis after resection of large bowel cancer. Br J Surg. 1985. 72:698–702.7. Blumberg D, Ramanathan RK. Treatment of colon and rectal cancer. J Clin Gastroenterol. 2002. 34:15–26.8. Minsky BD. Adjuvant therapy of resectable rectal cancer. Cancer Treat Rev. 2002. 28:181–188.9. Petrelli NJ. Will we ever succeed in resolving the adjuvant treatment dilemma in rectal cancer? J Surg Oncol. 2003. 82:79–83.10. Schnall MD, Furth EE, Rosato EF, Kressel HY. Rectal tumor stage: correlation of endorectal MR imaging and pathologic findings. Radiology. 1994. 190:709–714.11. Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999. 211:215–222.12. Beets-Tan RG, Beets GL, Borstlap AC, Oei TK, Teune TM, von Meyenfeldt MF, et al. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000. 25:533–541.13. Wallengren NO, Holtas S, Andren-Sandberg A, Jonsson E, Kristoffersson DT, McGill S. Rectal carcinoma: double-contrast MR imaging for preoperative staging. Radiology. 2000. 215:108–114.14. Urban M, Rosen HR, Holbling N, Feil W, Hochwarther G, Hruby W, et al. MR imaging for the preoperative planning of sphincter-saving surgery for tumors of the lower third of the rectum: use of intravenous and endorectal contrast materials. Radiology. 2000. 214:503–508.15. Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, et al. Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery. Lancet. 2001. 357:497–504.16. Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology. 2003. 227:371–377.17. Brown G, Radcliffe AG, Newcombe RG, Dallimore NS, Bourne MW, Williams GT. Preoperative assessment of prognostic factors in rectal cancer using high-resolution magnetic resonance imaging. Br J Surg. 2003. 90:355–364.18. Colon and Rectum. American Joint Committee on Cancer: AJCC cancer staging Handbook. 2002. 6th ed. New York: Springer-Verlag;127–138.19. Compton C, Fenoglio-Preiser CM, Pettigrew N, Fielding LP. Colorectal Working Group. American Joint Committee on Cancer Prognostic Factors Consensus Conference. Cancer. 2000. 88:1739–1757.20. Blomqvist L, Rubio C, Holm T, Machado M, Hindmarsh T. Rectal adenocarcinoma: assessment of tumour involvement of the lateral resection margin by MRI of resected specimen. Br J Radiol. 1999. 72:18–23.21. Kim NK, Kim MJ, Park JK, Park SI, Min JS. Preoperative staging of rectal cancer with MRI: accuracy and clinical usefulness. Ann Surg Oncol. 2000. 7:732–737.22. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003. 228:303–308.23. Goldstein NS, Turner JR. Pericolonic tumor deposits in patients with T3N+MO colon adenocarcinomas: markers of reduced disease free survival and intra-abdominal metastases and their implications for TNM classification. Cancer. 2000. 88:2228–2238.24. Harrison J, Haddad P, Maguire P. The impact of cancer on key relatives: a comparison of relative and patient concerns. Eur J Cancer. 1995. 31A:1736–1740.25. Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980. 67:439–442.26. Petersen VC, Baxter KJ, Love SB, Shepherd NA. Identification of objective pathological prognostic determinants and models of prognosis in Dukes' B colon cancer. Gut. 2002. 51:65–69.27. Bentzen SM, Balslev I, Pedersen M, Teglbjaerg PS, Hanberg-Soren sen F, Bone J, et al. Time to loco-regional recurrence after resection of Dukes' B and C colorectal cancer with or without adjuvant postoperative radiotherapy. A multivariate regression analysis. Br J Cancer. 1992. 65:102–107.28. Enker WE, Thaler HT, Cranor ML, Polyak T. Total mesorectal excision in the operative treatment of carcinoma of the rectum. J Am Coll Surg. 1995. 181:335–346.29. Beets-Tan RG, Beets GL, van der Hoop AG, Borstlap AC, van Boven H, Rongen MJ, et al. High-resolution magnetic resonance imaging of the anorectal region without an endocoil. Abdom Imaging. 1999. 24:576–581.30. Moriya Y, Hojo K, Sawada T, Koyama Y. Significance of lateral node dissection for advanced rectal carcinoma at or below the peritoneal reflection. Dis Colon Rectum. 1989. 32:307–315.31. Hida J, Yasutomi M, Fujimoto K, Maruyama T, Okuno K, Shindo K. Does lateral lymph node dissection improve survival in rectal carcinoma? Examination of node metastases by the clearing method. J Am Coll Surg. 1997. 184:475–480.32. Glass RE, Ritchie JK, Thompson HR, Mann CV. The results of surgical treatment of cancer of the rectum by radical resection and extended abdomino-iliac lymphadenectomy. Br J Surg. 1985. 72:599–601.33. Hojo K, Vernava AM 3rd, Sugihara K, Katumata K. Preservation of urine voiding and sexual function after rectal cancer surgery. Dis Colon Rectum. 1991. 34:532–539.34. Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997. 336:980–987.35. Stockholm Colorectal Cancer Study Group. Randomized study on preoperative radiotherapy in rectal carcinoma. Ann Surg Oncol. 1996. 3:423–430.36. Kaminsky-Forrett MC, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998. 42:935–941.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Preoperative Magnetic Resonance Imaging in Staging of Rectal Cancer
- Preoperative Radiological Staging of Rectal Cancer
- Prognostic Factors and Treatment of Recurrence after Local Excision of Rectal Cancer
- Recent Progress in Diagnosis and Treatment of Rectal Cancer
- Oncologic impact of pathologic response on clinical outcome after preoperative chemoradiotherapy in locally advanced rectal cancer