Surfactant Therapy for Neonatal Respiratory Distress Syndrome: A Review of Korean Experiences over 17 Years
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Kyunghee University, Seoul, Korea. baecw@khnmc.or.kr
- KMID: 1783141
- DOI: http://doi.org/10.3346/jkms.2009.24.6.1110
Abstract
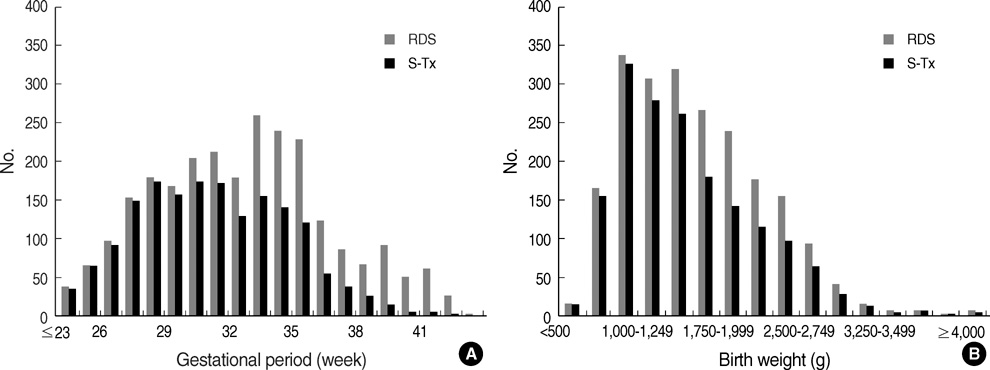
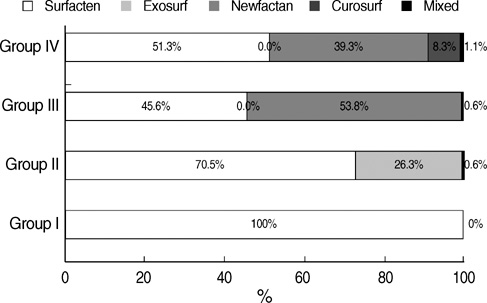
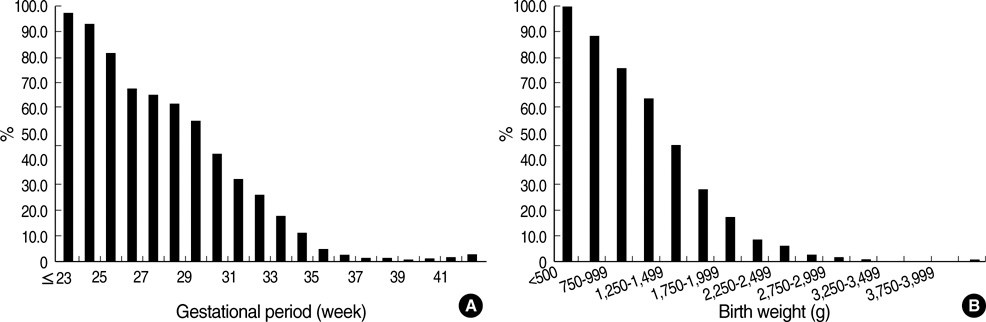
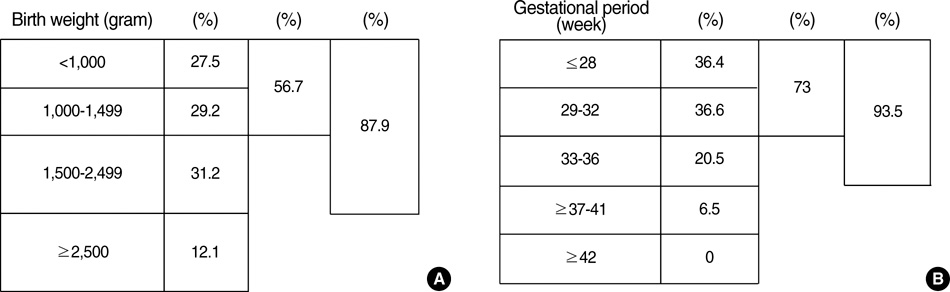
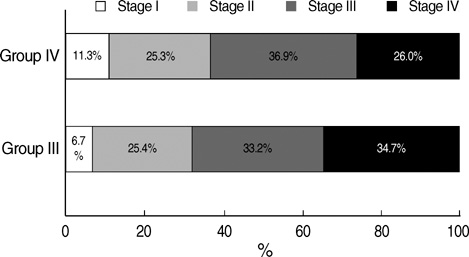
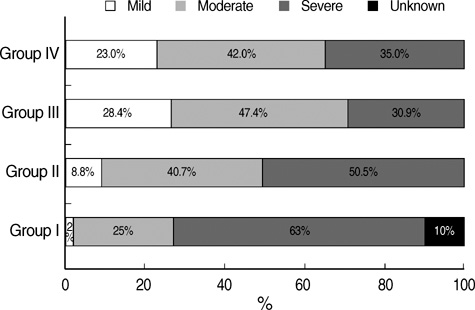
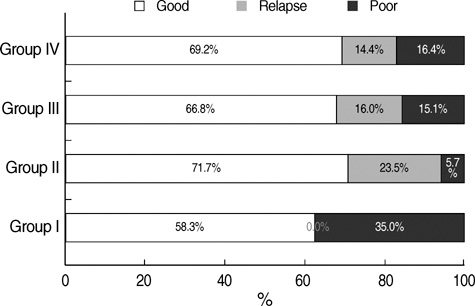
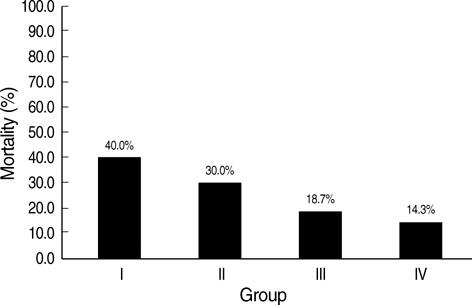
- We undertook a multi-hospital collective study to evaluate outcomes of neonatal respiratory distress syndrome (RDS) patients treated with pulmonary surfactant (PS) over 17 yr in Korea (Group I; 1990/91, Group II; 1996, Group III; 2002, and Group IV; 2007). There were 60 neonates in Group I (16 hospitals), 1,179 in Group II (64), 1,595 in Group III (62), and 1,921 in Group IV (57). We adopted Bomsel's classification to evaluate initial chest radiographic findings, categorized RDS severities, and classified response types to PS therapy. Almost all cases were treated using a single dose in Groups I and II, but 19.5% received multiple-dose therapy in Group IV. In Group IV, Bomsel's stages III and IV composed 62.9% and initial severities of mild, moderate, and severe RDS were 23.0%, 42.0%, and 35.0%. More infants showed good response in Groups II, III, and IV than in Group I (71.7%, 66.8%, and 69.2% vs. 58.3%). Complications and mortality rate were lower in Group IV than in Groups I, II, and III (mortality rate: 14.3% vs. 40.0%, 30.0%, and 18.7%). We conclude that PS therapy in neonates with RDS had a remarkable impact on improving clinical course and outcomes over 17 yr in Korea.
Keyword
MeSH Terms
-
Birth Weight
Female
Gestational Age
Humans
Infant, Newborn
Infant, Premature, Diseases/epidemiology/mortality/pathology/*therapy
Korea/epidemiology
Longitudinal Studies
Pregnancy
Pulmonary Surfactants/*therapeutic use
Questionnaires
Radiography, Thoracic
Respiratory Distress Syndrome, Newborn/epidemiology/mortality/pathology/*therapy
Treatment Outcome
Figure
Cited by 6 articles
-
Pulmonary Surfactant Replacement Therapy for Respiratory Distress Syndrome in Neonates: a Nationwide Epidemiological Study in Korea
Jeong Eun Shin, So Jin Yoon, Joohee Lim, Jungho Han, Ho Seon Eun, Min Soo Park, Kook In Park, Soon Min Lee
J Korean Med Sci. 2020;35(32):e253. doi: 10.3346/jkms.2020.35.e253.History of Pulmonary Surfactant Replacement Therapy for Neonatal Respiratory Distress Syndrome in Korea
Chong-Woo Bae, Chae Young Kim, Sung-Hoon Chung, Yong-Sung Choi
J Korean Med Sci. 2019;34(25):. doi: 10.3346/jkms.2019.34.e175.Development of a Synthetic Surfactant Using a Surfactant Protein-C Peptide Analog: In Vitro Studies of Surface Physical Properties
Chong-Woo Bae, Sung-Hoon Chung, Yong-Sung Choi
Yonsei Med J. 2016;57(1):203-208. doi: 10.3349/ymj.2016.57.1.203.New Medical Drugs for Care of Premature Infants
Chong-Woo Bae
J Korean Med Assoc. 2009;52(2):191-198. doi: 10.5124/jkma.2009.52.2.191.Admission of Term Infants to Neonatal Intensive Care Unit from Nursery
Jin Seok Park, Kee Hyun Cho, Heui Seung Jo, Sung-Il Cho, Gyu Young Chae, Moon Kyu Kim, Kyu Hyung Lee
Korean J Perinatol. 2014;25(4):246-256. doi: 10.14734/kjp.2014.25.4.246.Neonatal anesthesia: how we manage our most vulnerable patients
Si Ra Bang
Korean J Anesthesiol. 2015;68(5):434-441. doi: 10.4097/kjae.2015.68.5.434.
Reference
-
1. Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child. 1959. 97:517–523.
Article2. Fujiwara T, Maeta H, Chida S, Morita T, Watabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet. 1980. 1:55–59.
Article3. Namgung R, Lee C, Park KI, Han DG. Exogenous surfactant replacement therapy of hyaline membrane disease: a controlled clinical trial. J Korean Pediatr Soc. 1990. 33:22–35.4. Park CO, Lim BY, Yeo BG, Song JH, Sohn EK, Bae CW, Chung SJ, Ahn CI. Surfactant replacement therapy in neonatal respiratory distress syndrome. J Korean Pediatr Soc. 1991. 34:1211–1222.5. Bae CW, kwon YD, Ko SJ, Kim KS, Kim HM, Park WS, Byun SH, Son CS, Ahn HS, Lee SG, Chang YP, Chung YJ. Surfactant replacement therapy in neonates with respiratory distress syndrome: a collective evaluation of trials from 16 hospitals. J Korean Pediatr Soc. 1993. 36:244–265.6. Bae CW, Kim MH, Chun CS, Lee C, Moon SJ, Yoo BH, Lim BK, Lee SG, Choi YY, Byun SH, Choi AH, Pi SY, Han DG, Cho SH, Yoon JK. Surfactant replacement therapy in RDS: A collaborative study of multi-center trials in Korea. J Korean Soc Neonatol. 1997. 1:124–135.7. Bae CW, Kim YM. Surfactant therapy for neonatal respiratory distress syndrome: experience in Korea over 15 Years. Korean J Pediatr. 2004. 47:940–948.8. Bomsel F. Radiologic study of hyaline membrane disease: 110 cases. J Radiol Electrol Med Nucl. 1970. 51:259–268.9. Fujiwara T, Konishi M, Chida S, Okuyama K, Ogawa Y, Takeuchi Y, Nishida H, Kito H, Fujimura M, Nakamura H, Hashimoto T. Surfactant replacement therapy with a single postventilatory dose of a reconstituted bovine surfactant in preterm neonates with respiratory distress syndrome; final analysis of a multicenter, double-blind, randomized trial and comparison with similar trials. Pediatrics. 1990. 86:753–764.10. Fujiwara T, Konishi M, Chida S, Maeta H. Lachmann B, editor. Factors affecting the response to a postnasal single dose of a reconstituted bovine surfactant (surfactant-TA). Surfactant replacement therapy in neonatal and adult respiratory distress syndrome. 1988. Berlin: Springer-verlag;91–107.11. Sweet D, Bevilacqua G, Carnielli V, Greisen G, Plavka R, Didrik Saugstad O, Simeoni U, Speer CP, Valls-I-Soler A, Halliday H. European consensus guidelines on the management of neonatal respiratory distress syndrome. J Perinat Med. 2007. 35:175–186.
Article12. Jobe AH. Pulmonary surfactant therapy. N Engl J Med. 1993. 328:861–868.
Article13. Pramanik AK, Holtzman RB, Merritt TA. Surfactant replacement therapy for pulmonary disease. Pediatr Clin North Am. 1993. 40:913–936.14. Engle WA. American Academy of Pediatrics Committee on Fetusand Newborn. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics. 2008. 121:2419–2432.
Article15. Bae YM, Bae CW. The changes in the mortality rates of low birth weight infant and very low birth weight infant in Korea over the past 40 years. J Korean Med Sci. 2004. 19:27–31.
Article16. Bae CW. The changes in the birth and mortality rates of newborn in Korea. J Korean Med Assoc. 2006. 49:975–982.
Article17. Kim KS, Bae CW. Trends in survival rate for very low birth weight infants and extremely low birth weight infants in Korea, 1967-2007. Korean J Pediatr. 2008. 51:237–242.
Article18. Liechty EA, Donovan E, Purohit D, Gilhooly J, Feldman B, Noguchi A, Denson SE, Sehgal SS, Gross I, Stevens D, Ikegami M, Zachman RD, Carrier ST, Gunkel JH, Gold AJ. Reduction of neonatal mortality after multiple doses of bovine surfactant in low birth weight neonates with respiratory distress syndrome. Pediatrics. 1991. 88:19–28.