J Korean Med Sci.
2012 Sep;27(9):973-980. 10.3346/jkms.2012.27.9.973.
Assessing the Quality of Randomized Controlled Trials Published in the Journal of Korean Medical Science from 1986 to 2011
- Affiliations
-
- 1Department of Urology, Hanyang University College of Medicine, Seoul, Korea. swleepark@hanyang.ac.kr
- KMID: 1782127
- DOI: http://doi.org/10.3346/jkms.2012.27.9.973
Abstract
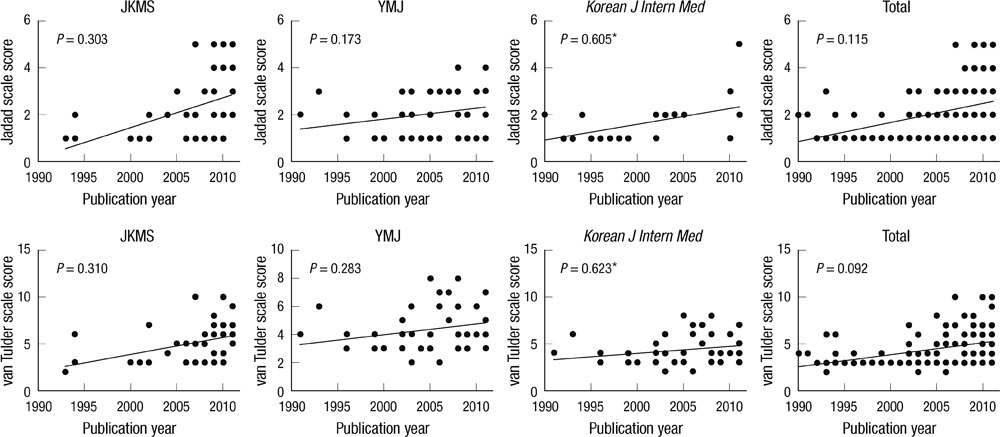
- Low quality clinical trials have a possibility to have errors in the process of deriving the results and therefore distort the study. Quality assessment of clinical trial is necessary in order to prevent any clinical application erroneous results is important. Randomized controlled trial (RCT) is a design for evaluate the effectiveness of medical procedure. This study was conducted by extracting the RCTs from the original articles published in the Journal of Korean Medical Science (JKMS) from 1986 to 2011 and conducting a qualitative analysis using three types of analysis tools: Jadad scale, van Tulder scale and Cochrane Collaboration risk of bias Tool. To compare the quality of articles of JKMS, quality analysis of the RCTs published in Yonsei Medical Journal (YMJ) and Korean Journal of Internal Medicine was also conducted. In the JKMS, YMJ and Korean Journal of Internal Medicine, the quantitative increase of RCT presented over time was observed but no qualitative improvement of RCT was observed over time. From the results of this study, it is required for the researchers to plan for and perform higher quality studies.
MeSH Terms
Figure
Cited by 1 articles
-
A Quality Analysis of Randomized Controlled Trials about Erectile Dysfunction
Jae Hoon Chung, Jeong Woo Lee, Jung Ki Jo, Kyu Shik Kim, Seung Wook Lee
World J Mens Health. 2013;31(2):157-162. doi: 10.5534/wjmh.2013.31.2.157.
Reference
-
1. Uetani K, Nakayama T, Ikai H, Yonemoto N, Moher D. Quality of reports on randomized controlled trials conducted in Japan: evaluation of adherence to the CONSORT statement. Intern Med. 2009. 48:307–313.2. Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996. 276:637–639.3. Jackson JL, Srinivasan M, Rea J, Fletcher KE, Kravitz RL. The validity of peer review in a general medicine journal. PLoS One. 2011. 6:e22475.4. Chung W, Lee KW, Hwang IH, Lee DH, Kim SY. Quality assessment of randomized controlled trials in the Journal of the Korean Academy of Family Medicine. Korean J Fam Med. 2009. 30:626–631.5. Liberati A, Himel HN, Chalmers TC. A quality assessment of randomized control trials of primary treatment of breast cancer. J Clin Oncol. 1986. 4:942–951.6. Autorino R, Borges C, White MA, Altunrende F, Perdona S, Haber GP, De Sio M, Khanna R, Stein RJ, Kaouk JH. Randomized clinical trials presented at the World Congress of Endourology: how is the quality of reporting? J Endourol. 2010. 24:2067–2073.7. Lim SM, Shin ES, Lee SH, Seo KH, Jung YM, Jang JE. Tools for assessing quality and risk of bias by levels of evidence. J Korean Med Assoc. 2011. 54:419–429.8. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996. 17:1–12.9. Moher D, Cook DJ, Jadad AR, Tugwell P, Moher M, Jones A, Pham B, Klassen TP. Assessing the quality of reports of randomised trials: implications for the conduct of meta-analyses. Health Technol Assess. 1999. 3:i–iv. 1–98.10. Kim SW, Choi YS, Ahn HS, Lee HY, Ahn DS, Lee YM. Quantity and quality assessment of randomized controlled trials published in five Korean medical journals, from 1980 to 2000. J Korean Acad Fam Med. 2004. 25:118–125.11. van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine (Phila Pa 1976). 2003. 28:1290–1299.12. Keech AC, Pike R, Granger RE, Gebski VJ. Interpreting the results of a clinical trial. Med J Aust. 2007. 186:318–319.13. Lee JY, Chung JH, Kang DH, Lee JW, Moon HS, Yoo TK, Choi HY, Lee SW. Quality assessment of randomized controlled trials published in the Korean Journal of Urology over the past 20 years. Korean J Urol. 2011. 52:642–646.14. Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002. 359:614–618.15. Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ. 2005. 330:1057–1058.16. Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001. 285:1987–1991.17. Armijo-Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Assessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract. 2012. 18:12–18.18. Clifford TJ, Barrowman NJ, Moher D. Funding source, trial outcome and reporting quality: are they related? Results of a pilot study. BMC Health Serv Res. 2002. 2:18.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessing the Quality of Randomized Controlled Urological Trials Conducted by Korean Medical Institutions
- Design and Conduct of Randomized Controlled Trials (RCTs)
- Quality Assessment of Non-Randomized Studies in the Korean Journal of Family Medicine
- Quality Assessment of Randomized Controlled Trials in the Journal of the Korean Academy of Family Medicine
- A Quality Analysis of Randomized Controlled Trials about Erectile Dysfunction