Korean J Lab Med.
2011 Oct;31(4):294-297. 10.3343/kjlm.2011.31.4.294.
Identification of Compound Heterozygous Mutation in a Korean Patient with Alpha 1-antitrypsin Deficiency
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. m91w95pf@snu.ac.kr
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea. dextro@snu.ac.kr
- KMID: 1781696
- DOI: http://doi.org/10.3343/kjlm.2011.31.4.294
Abstract
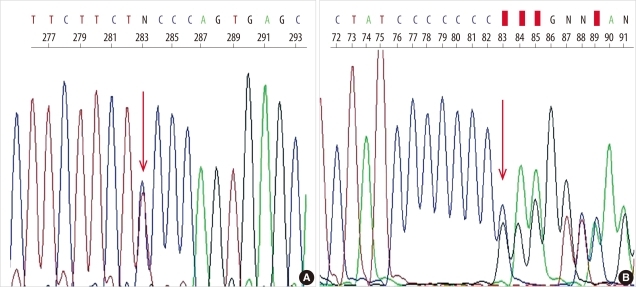
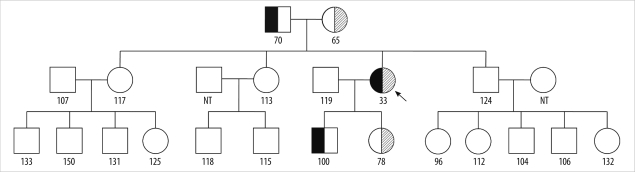
- Alpha 1-antitrypsin (AAT) deficiency is a genetic disorder that primarily affects the lungs and liver. While AAT deficiency is one of the most common genetic disorders in the Caucasian population, it is extremely rare in Asians. Here, we report the case of a 36-year-old Korean woman with AAT deficiency who visited the emergency department of our hospital for the treatment of progressive dyspnea that had begun 10 years ago. She had never smoked. Chest computed tomography revealed panlobular emphysema in both lungs, which suggested AAT deficiency. The serum AAT level was 33 mg/dL (reference interval: 90-200 mg/dL). Four exons of the SERPINA1 gene, which is responsible for AAT deficiency, and their flanking regions were analyzed by PCR-direct sequencing. The patient was found to have 1 missense mutation (c.230C>T, p.Ser77Phe; Siiyama) and 1 frameshift mutation (c.1158dupC, p.Glu387ArgfsX14; QOclayton). This is the first Korean case of AAT deficiency confirmed by genetic analysis and the second case of a compound heterozygote of Siiyama and QOclayton, the first case of which was reported from Japan.
MeSH Terms
-
Adult
Asian Continental Ancestry Group/*genetics
Base Sequence
Exons
Female
Frameshift Mutation
Heterozygote
Humans
Mutation, Missense
Pedigree
Pulmonary Emphysema/diagnosis/radiography
Republic of Korea
Sequence Analysis, DNA
Tomography, X-Ray Computed
alpha 1-Antitrypsin/genetics
alpha 1-Antitrypsin Deficiency/diagnosis/*genetics/radiography
Figure
Reference
-
1. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003; 168:818–900. PMID: 14522813.2. de Serres FJ. Worldwide racial and ethnic distribution of alpha1-antitrypsin deficiency: summary of an analysis of published genetic epidemiologic surveys. Chest. 2002; 122:1818–1829. PMID: 12426287.3. Ferrarotti I, Scabini R, Campo I, Ottaviani S, Zorzetto M, Gorrini M, et al. Laboratory diagnosis of alpha1-antitrypsin deficiency. Transl Res. 2007; 150:267–274. PMID: 17964515.
Article4. Seyama K, Nukiwa T, Takabe K, Takahashi H, Miyake K, Kira S. Siiyama (serine 53 [TCC] to phenylalanine 53 [TTC]). A new alpha 1-antitrypsin-deficient variant with mutation on a predicted conserved residue of the serpin backbone. J Biol Chem. 1991; 266:12627–12632. PMID: 1905728.
Article5. Brantly M, Lee JH, Hildesheim J, Uhm CS, Prakash UB, Staats BA, et al. alpha1-antitrypsin gene mutation hot spot associated with the formation of a retained and degraded null variant [corrected; erratum to be published]. Am J Respir Cell Mol Biol. 1997; 16:225–231. PMID: 9070606.
Article6. Huber R, Carrell RW. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989; 28:8951–8966. PMID: 2690952.7. Seyama K, Nukiwa T, Souma S, Shimizu K, Kira S. Alpha 1-antitrypsin-deficient variant Siiyama (Ser53[TCC] to Phe53[TTC]) is prevalent in Japan. Status of alpha 1-antitrypsin deficiency in Japan. Am J Respir Crit Care Med. 1995; 152:2119–2126. PMID: 8520784.
Article8. Faber JP, Poller W, Weidinger S, Kirchgesser M, Schwaab R, Bidlingmaier F, et al. Identification and DNA sequence analysis of 15 new alpha 1-antitrypsin variants, including two PI*Q0 alleles and one deficient PI*M allele. Am J Hum Genet. 1994; 55:1113–1121. PMID: 7977369.9. Carrell RW, Jeppsson JO, Laurell CB, Brennan SO, Owen MC, Vaughan L, et al. Structure and variation of human alpha 1-antitrypsin. Nature. 1982; 298:329–334. PMID: 7045697.10. P01009(A1AT_Human). Reviewed, UniProtKB/Swiss-Prot. Updated on May 2011. http://www.uniprot.org/uniprot/P01009.11. Miyahara N, Seyama K, Sato T, Fukuchi Y, Eda R, Takeyama H, et al. Compound heterozygosity for alpha-1-antitrypsin (S[iiyama] and QO[clayton]) in an Oriental patient. Intern Med. 2001; 40:336–340. PMID: 11334395.
Article12. Cooper DN, Ball EV, Stenson PD, Phillips AD, Shaw K, Mort ME. The human gene mutation database at the institute of medical genetics in cardiff. Updated on Jun 2005. http://www.hgmd.cf.ac.uk/ac/index.php.13. Husami A, editor. LOVD gene hompage. Updated on May 2010. https://research.cchmc.org/LOVD/home.php?select_db=SERPINA1.14. McPherson RA. McPherson RA, Pincus MR, editors. Specific proteins. Henry's clinical diagnosis and management by laboratory methods. 2007. 21st ed. Philadelphia, PA: WB Saunders;p. 236–241.
Article15. Okayama H, Brantly M, Holmes M, Crystal RG. Characterization of the molecular basis of the alpha 1-antitrypsin F allele. Am J Hum Genet. 1991; 48:1154–1158. PMID: 2035534.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Liver Involvement in Children with Alpha-1 Antitrypsin Deficiency: A Multicenter Study
- A compound heterozygous mutation in the FMO3 gene: the first pediatric case causes fish odor syndrome in Korea
- Neonatal Intrahepatic Cholestasis Caused by Citrin Deficiency in Korean Infants
- Plasmin and Its Inhibitors in the Lesional Skin of Pemphigus
- Early onset of colorectal cancer in a 13-year-old girl with Lynch syndrome