Korean J Lab Med.
2010 Dec;30(6):600-605. 10.3343/kjlm.2010.30.6.600.
Clinical Relevance of Elevated Levels of Serum Soluble Interleukin-2 Receptor alpha (sIL-2Ralpha) in Patients with Non-Hodgkin's Lymphoma
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University School of Medicine, Busan, Korea. mindcatch@hanmail.net, eylee@pusan.ac.kr
- 2Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 3Department of Pathology, Pusan National University School of Medicine, Busan, Korea.
- 4Medical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 1781666
- DOI: http://doi.org/10.3343/kjlm.2010.30.6.600
Abstract
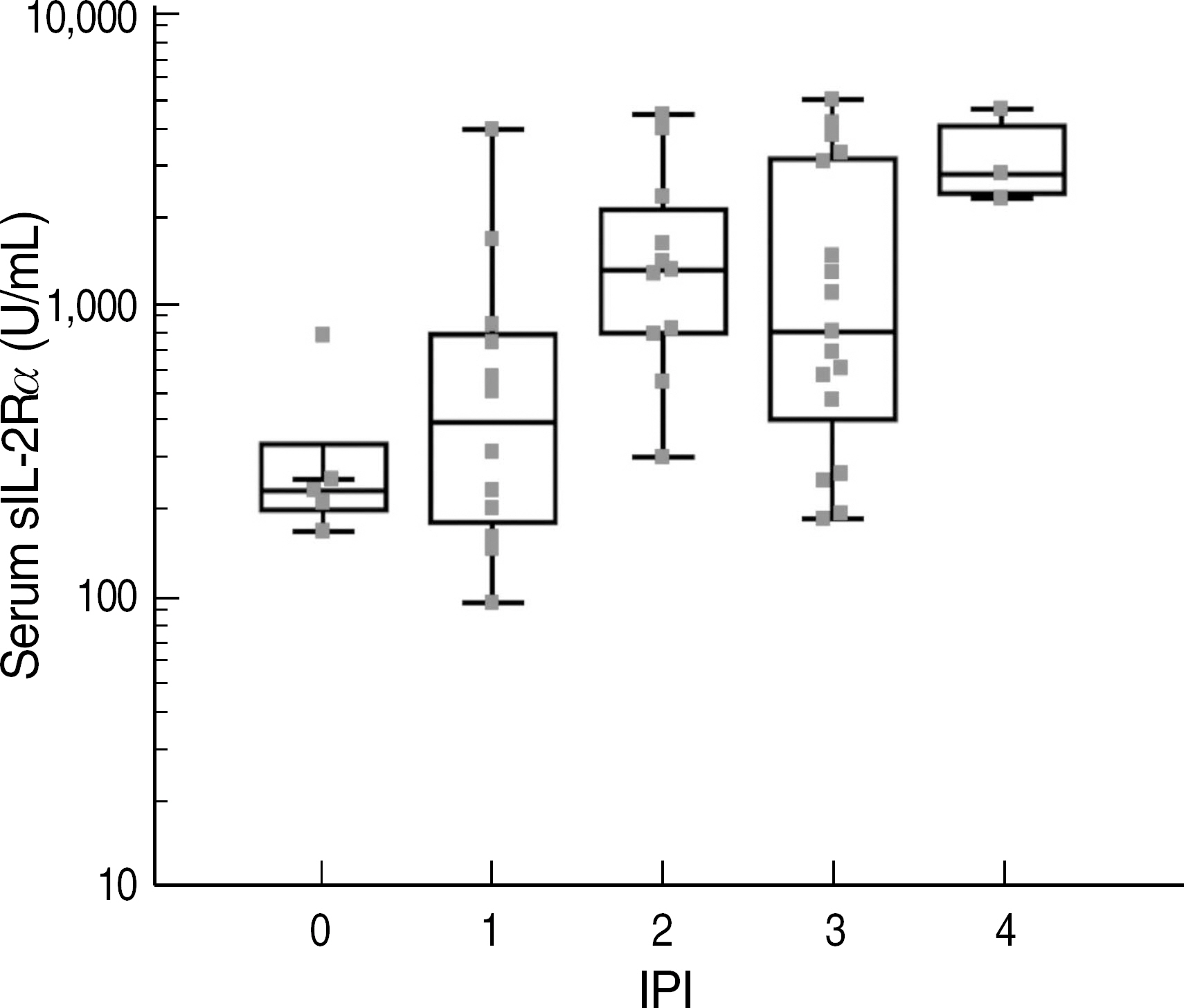
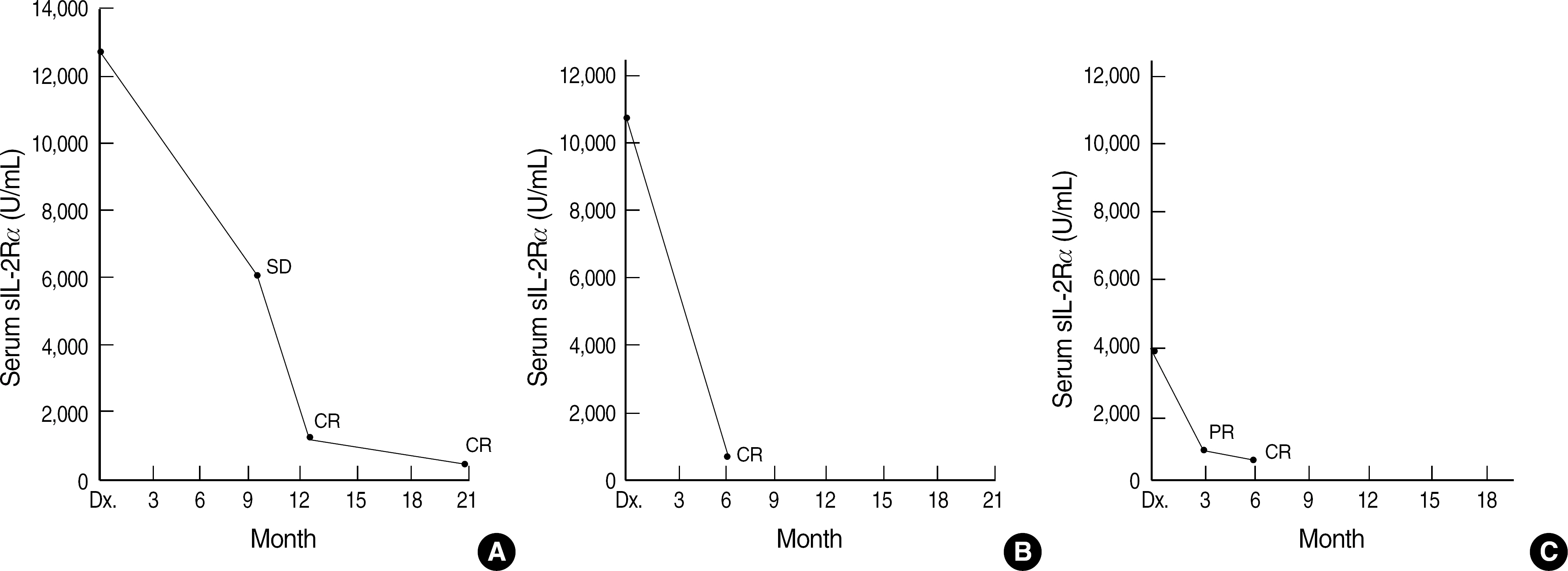
- Levels of soluble interleukin-2 receptor alpha (sIL-2Ralpha) are known to increase in the sera of patients with certain malignancies, including malignant lymphoma. This study aimed to assess the clinical significance of the sIL-2Ralpha level in non-Hodgkin's lymphoma (NHL). We used ELISA to measure the sIL-2Ralpha levels in 48 newly diagnosed and untreated patients with NHL and evaluated the correlation between the sIL-2Ralpha levels and clinical characteristics and the International Prognostic Index (IPI). We monitored serum sIL-2Ralpha in 7 patients to compare the changes in their clinical progress with these levels. High levels of serum sIL-2Ralpha (> or =2,000 U/mL) correlated well with parameters defining the high risk group according to the IPI, i.e., high tumor burden at diagnosis (stage III+IV) and lactate dehydrogenase > or =472 U/L. The levels were also associated with B symptoms, bone marrow involvement, and poor response to therapy. The sIL-2Ralpha level decreased during complete remission and was elevated during disease progression or relapse. A high level of sIL-2Ralpha was significantly associated with a low survival rate. These results suggest that serum sIL-2Ralpha might be useful as a biomarker for evaluating the prognosis of patients with NHL at the time of diagnosis and during therapy. A well-controlled, large-scale study is needed to clarify the clinical significance of sIL-2Ralpha in specific groups of NHL.
Keyword
MeSH Terms
Figure
Reference
-
1.Armitage JO., Weisenburger DD. New approach to classifying non-Hodgkin's lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin's Lymphoma Classification Project. J Clin Oncol. 1998. 16:2780–95.
Article2.Setoyama Y., Imai J., Ishikawa T., Sakaida H., Takaori-Kondo A., Kawamata S, et al. Usefulness of the measurement of serum soluble IL-2 receptor alpha chain levels in clinical monitoring of non-Hodgkin lymphoma. Rinsho Byori. 1994. 42:834–42.3.A predictive model for aggressive non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma Prognostic Factors Project. N Engl J Med. 1993. 329:987–94.4.Bien E., Balcerska A. Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers. 2008. 13:1–26.5.Gandhi MK., Lambley E., Burrows J., Dua U., Elliott S., Shaw PJ, et al. Plasma Epstein-Barr virus (EBV) DNA is a biomarker for EBV-positive Hodgkin's lymphoma. Clinical Cancer Research. 2006. 12:460–4.
Article6.Rubin LA., Galli F., Greene WC., Nelson DL., Jay G. The molecular basis for the generation of the human soluble interleukin 2 receptor. Cytokine. 1990. 2:330–6.
Article7.Waldmann TA. Anti-Tac (daclizumab, Zenapax) in the treatment of leukemia, autoimmune diseases, and in the prevention of allograft rejection: a 25-year personal odyssey. J Clin Immunol. 2007. 27:1–18.
Article8.Kitagawa JI., Hara T., Tsurumi H., Goto N., Kanemura N., Yoshikawa T, et al. Serum-soluble interleukin-2 receptor (sIL-2R) is an extremely strong prognostic factor for patients with peripheral T-cell lymphoma, unspecified (PTCL-U). J Cancer Res Clin Oncol. 2009. 135:53–59.
Article9.Oki Y., Kato H., Matsuo K., Kuwatsuka Y., Taji H., Yamamoto K, et al. Prognostic value of serum soluble interleukin-2 receptor level in patients with diffuse large B cell lymphoma, treated with CHOP- or RCHOP-based therapy. Leuk Lymphoma. 2008. 49:1345–51.
Article10.Wakao D., Murohashi I., Tominaga K., Yoshida K., Kishimoto K., Yagasaki F, et al. Serum thymidine kinase and soluble interleukin-2 receptor predict recurrence of malignant lymphoma. Ann Hematol. 2002. 81:140–6.
Article11.Goto H., Tsurumi H., Takemura M., Ino-Shimomura Y., Kasahara S., Sawada M, et al. Serum-soluble interleukin-2 receptor (sIL-2R) level determines clinical outcome in patients with aggressive non-Hodgkin's lymphoma: in combination with the International Prognostic Index. J Cancer Res Clin Oncol. 2005. 131:73–9.
Article12.Janik JE., Morris JC., Pittaluga S., McDonald K., Raffeld M., Jaffe ES, et al. Elevated serum-soluble interleukin-2 receptor levels in patients with anaplastic large cell lymphoma. Blood. 2004. 104:3355–7.
Article13.Yoshida S., Morii K. Serum concentrations of soluble interleukin-2 receptor in patients with malignant brain tumors. J Surg Oncol. 2000. 75:131–5.
Article14.Kaminska J., Kowalska M., Kotowicz B., Fuksiewicz M., Glogowski M., Wojcik E, et al. Pretreatment serum levels of cytokines and cytokine receptors in patients with non-small cell lung cancer, and correlations with clinicopathological features and prognosis. M-CSF - an independent prognostic factor. Oncology. 2006. 70:115–25.15.Witkowska AM. On the role of sIL-2R measurements in rheumatoid arthritis and cancers. Mediators Inflamm. 2005. 2005:121–130.
Article16.Sakata H., Murakami S., Hirayama R. Serum soluble interleukin-2 receptor (IL-2R) and immunohistochemical staining of IL-2R/Tac antigen in colorectal cancer. Int J Clin Oncol. 2002. 7:312–7.
Article17.Huang A., Quinn H., Glover C., Henderson DC., Allen-Mersh TG. The presence of interleukin-2 receptor alpha in the serum of colorectal cancer patients is unlikely to result only from T cell up-regulation. Cancer Immunol Immunother. 2002. 51:53–7.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Serum Anti-Acetylcholine Receptor Antibody, Interleukin-2 and Soluble Interleukin-2 Receptor Level in Myasthenia Gvavis
- Soluble Interleukin-2 Receptor(sIL-2R) Levels in Patients Tuberculous Pleurisy VS Nontuberculous Pleurisy
- Circulating Levels of Interleukin-6 and Soluble Interleukin-6 Receptor in Acute Asthma
- Levels of Serum Soluble Interleukin-2 Receptor in Patients with Chronic Myeloproliferative Disorders
- Can serum interleukin-2 receptor alpha predict lymph node metastasis in early gastric cancer?