Yonsei Med J.
2014 Jan;55(1):126-131. 10.3349/ymj.2014.55.1.126.
The Immunogenicity of a Single Dose of Hepatitis A Virus Vaccines (Havrix(R) and Epaxal(R)) in Korean Young Adults
- Affiliations
-
- 1Department of Preventive Medicine, Eulji University School of Medicine, Daejeon, Korea. moranki@naver.com
- 2Department of Preventive Medicine & Public Health, College of Medicine, Kwandong University, Gangneung, Korea.
- 3Department of Preventive Medicine, School of Medicine, Inje University, Busan, Korea.
- 4Department of Preventive Medicine, College of Medicine, Konyang University, Daejeon, Korea.
- 5Department of Preventive Medicine, School of Medicine, Keimyung University, Daegu, Korea.
- 6Department of Preventive Medicine, Institute for Poverty Alleviation & International Development, Yonsei University Wonju College of Medicine, Wonju, Korea.
- 7Deparment of Preventive Medicine, Medical College Soonchunhyang University, Cheonan, Korea.
- 8Department of Preventive Medicine, Wonkwang University Medical School, Iksan, Korea.
- KMID: 1779896
- DOI: http://doi.org/10.3349/ymj.2014.55.1.126
Abstract
- PURPOSE
Assessing the immunogenicity of a single dose of hepatitis A virus (HAV) vaccines is important because some people receive only a single dose. However, previous studies have shown variable results and have not examined the effects of demographic characteristics other than gender. This study was performed to examine the immunogenicity of a single dose of HAV vaccine according to the vaccine type and demographic characteristics in young adults.
MATERIALS AND METHODS
Seronegative medical school students were randomly allocated to receive either Havrix or Epaxal.
RESULTS
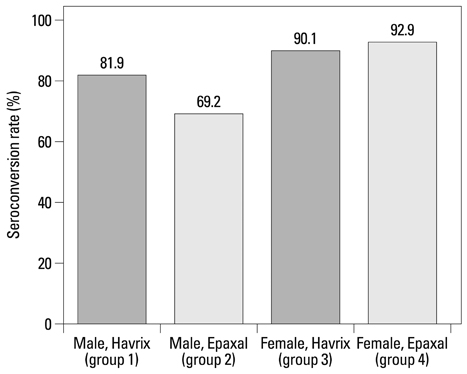
After approximately 11 months, the seroconversion rate in 451 participants was 80.7%. In men, the Havrix group showed a significantly higher seroconversion rate (81.9%) than the Epaxal group (69.2%), whereas both vaccine groups showed similarly high immunogenicity in women (Havrix: 90.1%, Epaxal: 92.9%; P for interaction=0.062). According to the results of a multivariate analysis, Epaxal showed significantly lower immunogenicity than Havrix only in men. Age, obesity, drinking, smoking, and follow-up time did not significantly affect seroconversion in either gender.
CONCLUSION
The seroconversion rate of single-dose HAV vaccines was low in men, particularly in those who received Epaxal. Our results suggest that gender effects should be considered when comparing the immunogenicity of different HAV vaccines.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee D, Cho YA, Park Y, Hwang JH, Kim JW, Kim NY, et al. Hepatitis a in Korea: epidemiological shift and call for vaccine strategy. Intervirology. 2008; 51:70–74.
Article2. WHO position paper on hepatitis A vaccines-June 2012. Wkly Epidemiol Rec. 2012; 87:261–276.3. Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, et al. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008; 198:1776–1782.
Article4. López EL, Contrini MM, Mistchenko A, Debbag R. Long-term immunity after two doses of inactivated hepatitis A vaccine, in Argentinean children. Pediatr Infect Dis J. 2010; 29:568–570.
Article5. Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol. 2011; 83:1885–1891.
Article6. Landry P, Tremblay S, Darioli R, Genton B. Inactivated hepatitis A vaccine booster given >/=24 months after the primary dose. Vaccine. 2000; 19:399–402.
Article7. D'Acremont V, Herzog C, Genton B. Immunogenicity and safety of a virosomal hepatitis A vaccine (Epaxal) in the elderly. J Travel Med. 2006; 13:78–83.8. Bovier PA, Farinelli T, Loutan L. Interchangeability and tolerability of a virosomal and an aluminum-adsorbed hepatitis A vaccine. Vaccine. 2005; 23:2424–2429.
Article9. Ambrosch F, Finkel B, Herzog C, Koren A, Kollaritsch H. Rapid antibody response after vaccination with a virosomal hepatitis a vaccine. Infection. 2004; 32:149–152.
Article10. Holzer BR, Hatz C, Schmidt-Sissolak D, Glück R, Althaus B, Egger M. Immunogenicity and adverse effects of inactivated virosome versus alum-adsorbed hepatitis A vaccine: a randomized controlled trial. Vaccine. 1996; 14:982–986.
Article11. Bovier PA, Althaus B, Glueck R, Chippaux A, Loutan L. Tolerance and immunogenicity of the simultaneous administration of virosome hepatitis A and yellow fever vaccines. J Travel Med. 1999; 6:228–233.
Article12. Force IOT. The Asia-Pacific perspective: Redefining obesity and its treatment IOTF, Health Communications Australia Pty Ltd. Brisbane: World Health Organization/International Association for the Study of Obesity;2000.13. Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. AUDIT: The Alcohol Use Disorders Identification Test. Guidelines for Use in Primary Care. 2nd ed. Geneva: World Health Organization;2001.14. Maiwald H, Jilg W, Bock HL, Löscher T, Sonnenburg F. Long-term persistence of anti-HAV antibodies following active immunization with hepatitis A vaccine. Vaccine. 1997; 15:346–348.
Article15. Reuman PD, Kubilis P, Hurni W, Brown L, Nalin D. The effect of age and weight on the response to formalin inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine. 1997; 15:1157–1161.
Article16. Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Clin Liver Dis. 2004; 8:283–300.
Article17. Jeon GS, Lee HY. Associated Factors of Binge Drinking and Problem Drinking among Korean Men and Women. Korean J Health Educ Promot. 2010; 27:91–103.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reappraisal of the Immunogenicity and Safety of Three Hepatitis A Vaccines in Adolescents
- Immunogenicity of a Recombinant Hepatitis B Virus Vaccine Compared with a Plasma-derived Hepatitis B Vaccine and of Vaccination Schedules in Neonates
- Hepatitis A Vaccine
- Effects of Addition of Sugars on the Stability of Hepatitis B Virus Vaccine
- Current Status and Vaccine Indication for Hepatitis A Virus Infection in Korea