Yonsei Med J.
2011 Mar;52(2):227-233. 10.3349/ymj.2011.52.2.227.
The Decellularized Vascular Allograft as an Experimental Platform for Developing a Biocompatible Small-Diameter Graft Conduit in a Rat Surgical Model
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 2Department of Biochemistry and Molecular Biology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jwlee@amc.seoul.kr
- 4Department of Thoracic and Cardiovascular Surgery, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea.
- 5Department of Thoracic and Cardiovascular Surgery, Yangsan Hospital, Pusan National University School of Medicine, Busan, Korea.
- KMID: 1779656
- DOI: http://doi.org/10.3349/ymj.2011.52.2.227
Abstract
- PURPOSE
The present study was aimed to assess the feasibility of using decellularized aortic allograft in a rat small animal surgical model for conducting small diameter vascular tissue engineering research.
MATERIALS AND METHODS
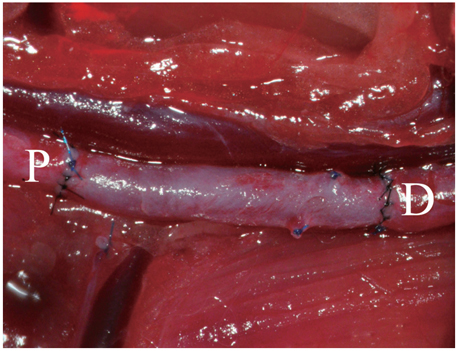
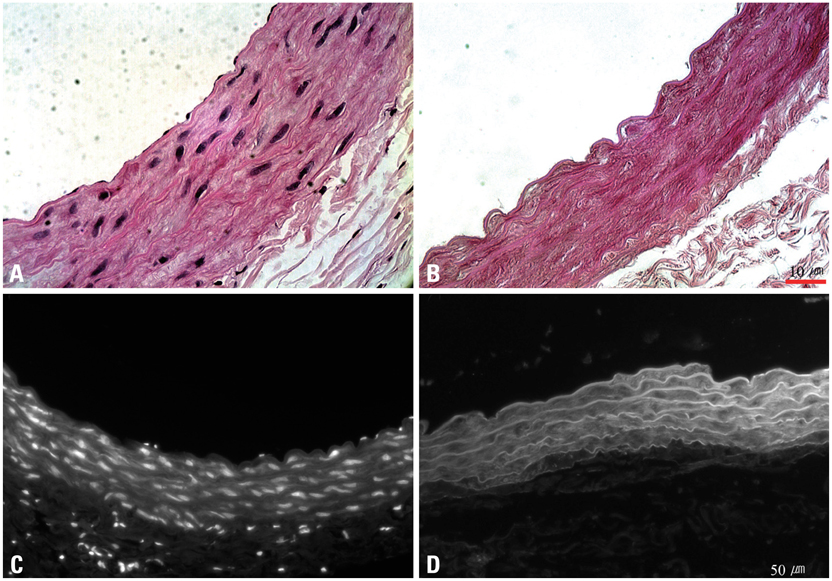
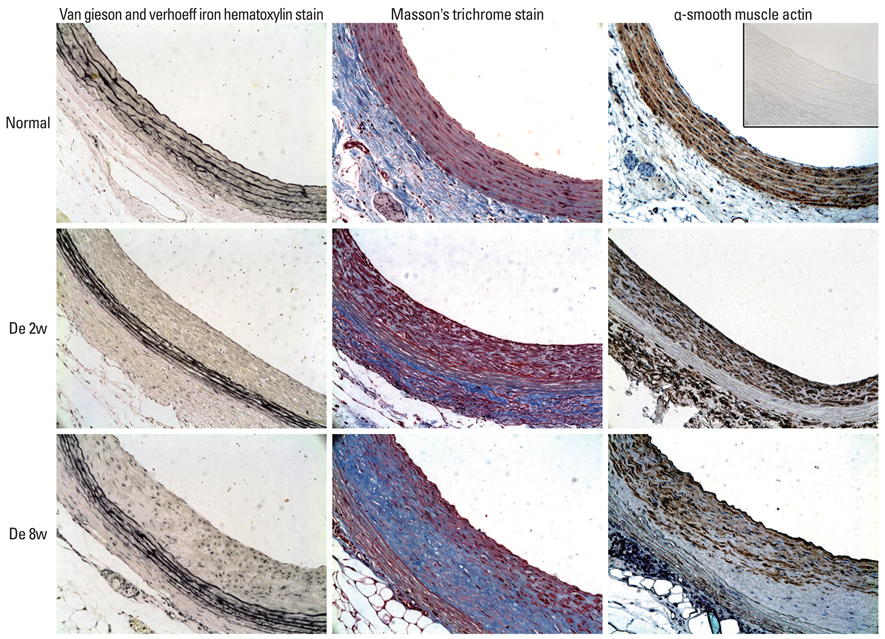
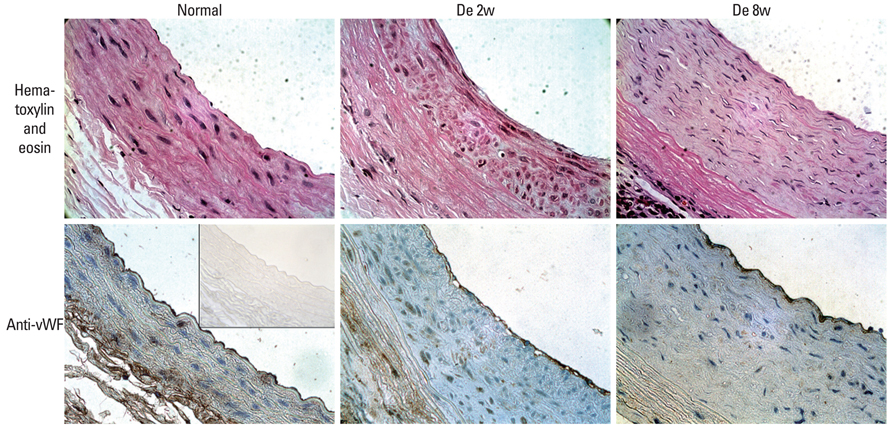
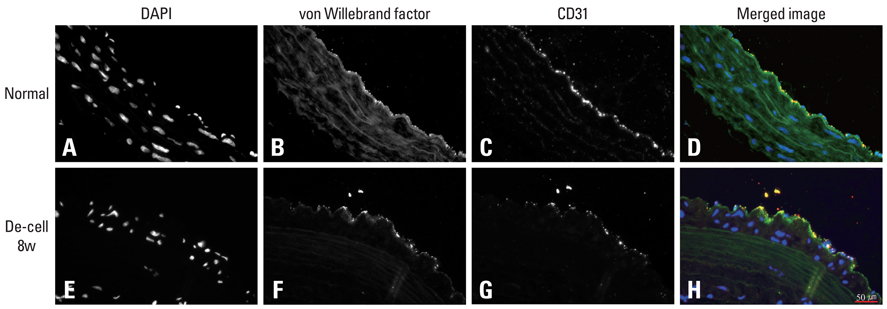
Decellularized aortic allografts were infra-renally implanted in 12 Sprague-Dawley (SD) adult rats. The conduits were harvested at 2 (n = 6) and 8 weeks (n = 6), and assessed by hematoxylin and eosin (H&E), van Gieson, Masson Trichrome staining, and immunohistochemistry for von Willebrand factor, CD 31+, and actin.
RESULTS
Consistent, predictable, and reproducible results were produced by means of a standardized surgical procedure. All animals survived without major complications. Inflammatory immune reaction was minimal, and there was no evidence of aneurysmal degeneration or rupture of the decellularized vascular implants. However, the aortic wall appeared thinner and the elastic fibers in the medial layer showed decreased undulation compared to the normal aorta. There was also minimal cellular repopulation of the vascular media. The remodeling appeared progressive from 2 to 8 weeks with increased intimal thickening and accumulation of both collagen and cells staining for actin. Although the endothelial like cells appeared largely confluent at 8 weeks, they were not as concentrated in appearance as in the normal aorta.
CONCLUSION
The results showed the present rat animal model using decellularized vascular allograft implants to be a potentially durable and effective experimental platform for conducting further research on small diameter vascular tissue engineering.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The principles of tissue engineering and its recent advances and future prospects
Woo Seob Kim
J Korean Med Assoc. 2014;57(2):145-154. doi: 10.5124/jkma.2014.57.2.145.
Reference
-
1. Fitzgibbon GM, Kafka HP, Leach AJ, Keon WJ, Hooper GD, Burton JR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol. 1996. 28:616–626.
Article2. Taylor LM Jr, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990. 11:193–205.
Article3. Veith FJ, Gupta SK, Ascer E, White-Flores S, Samson RH, Scher LA, et al. Six-year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986. 3:104–114.
Article4. Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur J Vasc Endovasc Surg. 2004. 27:357–362.
Article5. Kashyap VS, Ahn SS, Quinones-Baldrich WJ, Choi BU, Dorey F, Reil TD, et al. Infrapopliteal-lower extremity revascularization with prosthetic conduit: a 20-year experience. Vasc Endovascular Surg. 2002. 36:255–262.
Article6. Coburn MC, Carney WI Jr. Comparison of basilic vein and polytetrafluoroethylene for brachial arteriovenous fistula. J Vasc Surg. 1994. 20:896–902.
Article7. Kherlakian GM, Roedersheimer LR, Arbaugh JJ, Newmark KJ, King LR. Comparison of autogenous fistula versus expanded polytetrafluoroethylene graft fistula for angioaccess in hemodialysis. Am J Surg. 1986. 152:238–243.
Article8. Bayne K. American Physiological Society. Revised Guide for the Care and Use of Laboratory Animals available. Physiologist. 1996. 39:199208–211.9. Isenberg BC, Williams C, Tranquillo RT. Small-diameter artificial arteries engineered in vitro. Circ Res. 2006. 98:25–35.
Article10. Conte MS. The ideal small arterial substitute: a search for the Holy Grail? FASEB J. 1998. 12:43–45.
Article11. Quiñones-Baldrich WJ, Prego AA, Ucelay-Gomez R, Freischlag JA, Ahn SS, Baker JD, et al. Long-term results of infrainguinal revascularization with polytetrafluoroethylene: a ten-year experience. J Vasc Surg. 1992. 16:209–217.
Article12. Ritter EF, Fata MM, Rudner AM, Klitzman B. Heparin bonding increases patency of long microvascular prostheses. Plast Reconstr Surg. 1998. 101:142–146.
Article13. Wong G, Li JM, Hendricks G, Eslami MH, Rohrer MJ, Cutler BS. Inhibition of experimental neointimal hyperplasia by recombinant human thrombomodulin coated ePTFE stent grafts. J Vasc Surg. 2008. 47:608–615.
Article14. Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004. 40:146–153.
Article15. Martin ND, Schaner PJ, Tulenko TN, Shapiro IM, Dimatteo CA, Williams TK, et al. In vivo behavior of decellularized vein allograft. J Surg Res. 2005. 129:17–23.16. Wagner E, Roy R, Marois Y, Douville Y, Guidoin R. Fresh venous allografts in peripheral arterial reconstruction in dogs. Effects of histocompatibility and of short-term immunosuppression with cyclosporine A and mycophenolate mofetil. J Thorac Cardiovasc Surg. 1995. 110:1732–1744.17. Allaire E, Guettier C, Bruneval P, Plissonnier D, Michel JB. Cell-free arterial grafts: morphologic characteristics of aortic isografts, allografts, and xenografts in rats. J Vasc Surg. 1994. 19:446–456.
Article18. Allaire E, Bruneval P, Mandet C, Becquemin JP, Michel JB. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997. 122:73–81.
Article19. Iaffaldano RA, Lewis BE, Johnson SA, Piffare R, McKiernan TL. Patency of cryopreserved saphenous vein grafts as conduits for coronary artery bypass surgery. Chest. 1995. 108:725–729.
Article20. Lopez-Soler RI, Brennan MP, Goyal A, Wang Y, Fong P, Tellides G, et al. Development of a mouse model for evaluation of small diameter vascular grafts. J Surg Res. 2007. 139:1–6.
Article21. Atoui R, Asenjo JF, Duong M, Chen G, Chiu RC, Shum-Tim D. Marrow stromal cells as universal donor cells for myocardial regenerative therapy: their unique immune tolerance. Ann Thorac Surg. 2008. 85:571–579.
Article22. Traktuev DO, Tsokolaeva ZI, Shevelev AA, Talitskiy KA, Stepanova VV, Johnstone BH, et al. Urokinase gene transfer augments angiogenesis in ischemic skeletal and myocardial muscle. Mol Ther. 2007. 15:1939–1946.
Article23. Pascual G, Martínez S, Rodríguez M, Serrano N, Bellón JM, Buján J. Patency and structural changes in cryopreserved arterial grafts used as vessel substitutes in the rat. J Surg Res. 2005. 124:297–304.
Article24. Lesauskaite V, Tanganelli P, Sassi C, Neri E, Diciolla F, Ivanoviene L, et al. Smooth muscle cells of the media in the dilatative pathology of ascending thoracic aorta: morphology, immunoreactivity for osteopontin, matrix metalloproteinases, and their inhibitors. Hum Pathol. 2001. 32:1003–1011.
Article25. Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, et al. A murine model of thoracic aortic aneurysms. J Surg Res. 2003. 115:157–163.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heparin Immobilization of Tissue Engineered Xenogeneic Small Diameter Arterial Scaffold Improve Endothelialization
- Vitrified Human Umbilical Arteries as Potential Grafts for Vascular Tissue Engineering
- Cuff Technique for Small-Diameter Vascular Grafts in the Systemic Arterial Circulation of the Rat
- Effectiveness and Biocompatibility of Decellularized Nerve Graft Using an In Vivo Rat Sciatic Nerve Model
- Clinical applicability of autologous great saphenous vein for living donor liver transplantation