J Korean Med Sci.
2010 Feb;25(2):240-244. 10.3346/jkms.2010.25.2.240.
Pulmonary Toxicity after a Quick Course of Combinatorial Vincristine, Bleomycin, and Cisplatin Neoadjuvant Chemotherapy in Cervical Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, East-West Neo Medical Center, Kyung Hee University, Seoul, Korea. kgo02@hanmail.net
- 2Department of Radiology, East-West Neo Medical Center, Kyung Hee University, Seoul, Korea.
- 3Department of Pathology, East-West Neo Medical Center, Kyung Hee University, Seoul, Korea.
- KMID: 1779254
- DOI: http://doi.org/10.3346/jkms.2010.25.2.240
Abstract
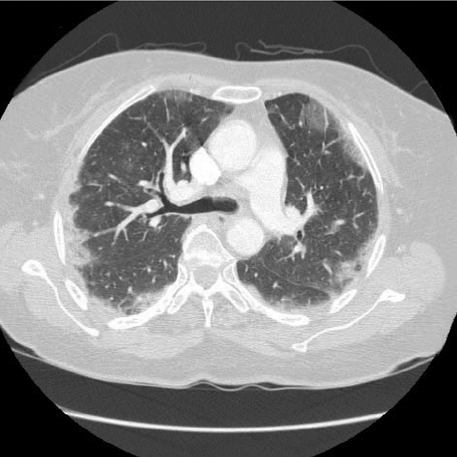
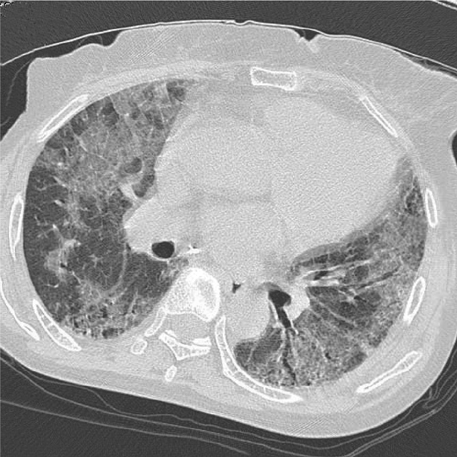
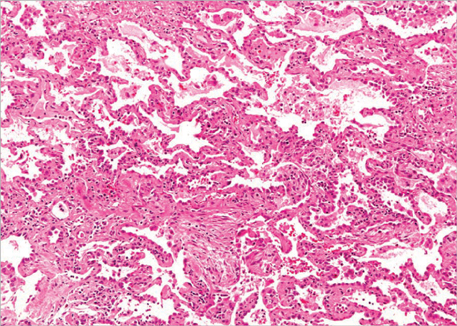
- Pulmonary toxicity is one of the most serious adverse effects associated with a quick course of vincristine, bleomycin, and cisplatin neoadjuvant chemotherapy (NAC-VBP). The aim of this study was to evaluate pulmonary toxicity related to a quick course NAC-VBP. A total of consecutive 61 patients, who underwent at most 3 cycles of NAC-VBP every 10 days in the International Federation of Gynecology and Obstetrics (FIGO) stage IB-IIB cervical cancer from 1995 to 2007, were retrospectively analyzed. Of the 61 study subjects, 7 (11.5%) were identified to have pulmonary toxicity and 2 (3.3%) died of pulmonary fibrosis progression despite aggressive treatment and the use of a multidisciplinary approach. No factor predisposing pulmonary toxicity was identified. Initial symptoms were non-specific, but bronchiolitis obliterans organizing pneumonia and interstitial pneumonitis were characteristic findings by high-resolution computed tomography of the chest. The benefit of steroid therapy was uncertain and was associated with steroid-induced diabetes mellitus requiring insulin therapy in two patients. Fatal pulmonary toxicity is a major concern of a quick course NAC-VBP. In conclusion, these patients require special monitoring for bleomycin-induced pulmonary toxicity.
MeSH Terms
-
Aged
Antineoplastic Combined Chemotherapy Protocols/administration & dosage/*adverse effects/therapeutic use
Bleomycin/administration & dosage/*adverse effects/therapeutic use
Cisplatin/administration & dosage/*adverse effects/therapeutic use
Female
Humans
Lung Diseases/*chemically induced/pathology
Middle Aged
*Neoadjuvant Therapy
Pulmonary Fibrosis/chemically induced/mortality/pathology
Retrospective Studies
Tomography, X-Ray Computed
Uterine Cervical Neoplasms/complications/*drug therapy
Vincristine/administration & dosage/*adverse effects/therapeutic use
Bleomycin
Cisplatin
Vincristine
Figure
Reference
-
1. Shin HR, Jung KW, Won YJ, Park JG. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.
Article2. Kim DS, Moon H, Hwang YY, Cho SH. Preoperative adjuvant chemotherapy in the treatment of cervical cancer stage Ib, IIa, and IIb with bulky tumor. Gynecol Oncol. 1988. 29:321–332.
Article3. Weiner SA, Aristizabal S, Alberts DS, Surwit EA, Deatherage-Deuser K. A phase II trial of mitomycin, vincristine, bleomycin, and cisplatin (MOBP) as neoadjuvant therapy in high-risk cervical carcinoma. Gynecol Oncol. 1988. 30:1–6.
Article4. Panici PB, Scambia G, Baiocchi G, Greggi S, Ragusa G, Gallo A, Conte M, Battaglia F, Laurelli G, Rabitti C. Neoadjuvant chemotherapy and radical surgery in locally advanced cervical cancer. Prognostic factors for response and survival. Cancer. 1991. 67:372–379.
Article5. Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, Snaidas L, Vighi S, Gomez Rueda N, di Paola G. Long-term follow-up of the first randomized trial using neoadjuvant chemotherapy in stage Ib squamous carcinoma of the cervix: the final results. Gynecol Oncol. 1997. 67:61–69.
Article6. Lai CH, Hsueh S, Chang TC, Tseng CJ, Huang KG, Chou HH, Chen SM, Chang MF, Shum HC. Prognostic factors in patients with bulky stage IB or IIA cervical carcinoma undergoing neoadjuvant chemotherapy and radical hysterectomy. Gynecol Oncol. 1997. 64:456–462.
Article7. Chang TC, Lai CH, Hong JH, Hsueh S, Huang KG, Chou HH, Tseng CJ, Tsai CS, Chang JT, Lin CT, Chang HH, Chao PJ, Ng KK, Tang SG, Soong YK. Randomized trial of neoadjuvant cisplatin, vincristine, bleomycin, and radical hysterectomy versus radiation therapy for bulky stage IB and IIA cervical cancer. J Clin Oncol. 2000. 18:1740–1747.
Article8. Porzio G, Ficorella C, Toro G, Paris I, Ricevuto E, Marchetti P. Short-term weekly neoadjuvant chemotherapy in the treatment of locally advanced cervical cancer. Tumori. 2001. 87:25–26.
Article9. Huang HJ, Chang TC, Hong JH, Tseng CJ, Chou HH, Huang KG, Lai CH. Prognostic value of age and histologic type in neoadjuvant chemotherapy plus radical surgery for bulky (>/=4 cm) stage IB and IIA cervical carcinoma. Int J Gynecol Cancer. 2003. 13:204–211.10. Singh KC, Agarwal A, Agarwal S, Rajaram S, Goel N, Agarwal N. 'Quick course' neoadjuvant chemotherapy with cisplatin, bleomycin and vincristine in advanced cervical cancer. Gynecol Obstet Invest. 2004. 58:109–113.
Article11. World Health Organization. WHO handbook for reporting results of cancer treatment: WHO offset publication. 1979. Geneva, Switzerland:12. Benedetti-Panici P, Greggi S, Colombo A, Amoroso M, Smaniotto D, Giannarelli D, Amunni G, Raspagliesi F, Zola P, Mangioni C, Landoni F. Neoadjuvant chemotherapy and radical surgery versus exclusive radiotherapy in locally advanced squamous cell cervical cancer: results from the Italian multicenter randomized study. J Clin Oncol. 2002. 20:179–188.
Article13. Behtash N, Mehrdad N. Cervical cancer: screening and prevention. Asian Pac J Cancer Prev. 2006. 7:683–686.14. Eddy GL, Bundy BN, Creasman WT, Spirtos NM, Mannel RS, Hannigan E, O'Connor D. Treatment of ("bulky") stage IB cervical cancer with or without neoadjuvant vincristine and cisplatin prior to radical hysterectomy and pelvic/para-aortic lymphadenectomy: a phase III trial of the gynecologic oncology group. Gynecol Oncol. 2007. 106:362–369.
Article15. Kreisman H, Wolkove N. Pulmonary toxicity of antineoplastic therapy. Semin Oncol. 1992. 19:508–520.16. Adamson IY. Drug-induced pulmonary fibrosis. Environ Health Perspect. 1984. 55:25–36.
Article17. Azambuja E, Fleck JF, Batista RG, Menna Barreto SS. Bleomycin lung toxicity: who are the patients with increased risk? Pulm Pharmacol Ther. 2005. 18:363–366.
Article18. Sleijfer S. Bleomycin-induced pneumonitis. Chest. 2001. 120:617–624.
Article19. Simpson AB, Paul J, Graham J, Kaye SB. Fatal bleomycin pulmonary toxicity in the west of Scotland 1991-95: a review of patients with germ cell tumours. Br J Cancer. 1998. 78:1061–1066.
Article20. Levi JA, Raghavan D, Harvey V, Thompson D, Sandeman T, Gill G, Stuart-Harris R, Snyder R, Byrne M, Kerestes Z. Australasian Germ Cell Trial Group. The importance of bleomycin in combination chemotherapy for good-prognosis germ cell carcinoma. J Clin Oncol. 1993. 11:1300–1305.21. Sardi JE. Phase II trial with neoadjuvant chemotherapy. Gynecol Oncol. 1996. 62:321–322.22. Bloss JD, Lucci JA 3rd, DiSaia PJ, Manetta A, Schiano MA, Ramsinghani N, Berman ML. A phase II trial of neoadjuvant chemotherapy prior to radical hysterectomy and/or radiation therapy in the management of advanced carcinoma of the uterine cervix. Gynecol Oncol. 1995. 59:105–110.
Article23. Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004. 25:53–64.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Neoadjuvant PI(Cisplatin, Ifosfamide) and PAIB(Cisplatin, Adriamycin, Ifosfamide, Bleomycin) in the Cervical Cancer
- Neoadjuvant chemotherapy in stage IB2 cervical cancer
- Neoadjuvant chemotherapy with mitomycin-C, vincristine and cisplatin (MVC) for patients with loco-regionally advanced cervical carcinoma
- Clinical Significance of UFT-Cisplatin Combined Neoadjuvant Chemotherpy in Uterine Cervical Cancer
- Treatment with cisplatin, bleomycin, etoposide combination chemotherapy for a case of Wilms' tumor which relapsed at lung and liver