J Korean Med Sci.
2010 Mar;25(3):476-480. 10.3346/jkms.2010.25.3.476.
A Case of Granulocyte-Colony Stimulating Factor-Producing Hepatocellular Carcinoma Confirmed by Immunohistochemistry
- Affiliations
-
- 1Department of Internal Medicine, Matsumoto Medical Center, Matsumoto, Japan. joshita@shinshu-u.ac.jp
- 2Department of Internal Medicine, Division of Gastroenterology and Hepatology, Shinshu University School of Medicine, Matsumoto, Japan.
- 3Department of Laboratory Medicine, Matsumoto Medical Center, Matsumoto, Japan.
- 4Department of Surgery, Matsumoto Medical Center, Matsumoto, Japan.
- KMID: 1778047
- DOI: http://doi.org/10.3346/jkms.2010.25.3.476
Abstract
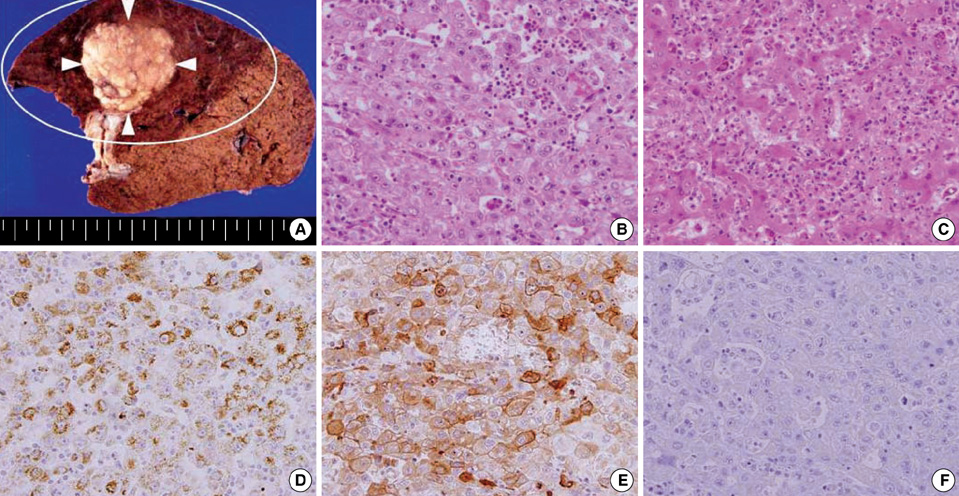
- Granulocyte-colony stimulating factor (G-CSF) is a naturally occurring glycoprotein that stimulates the proliferation and maturation of precursor cells in the bone marrow into fully differentiated neutrophils. Several reports of G-CSF-producing malignant tumors have been published, but scarcely any in the hepatobiliary system, such as in hepatocellular carcinoma (HCC). Here, we encountered a 69-yr-old man with a hepatic tumor who had received right hepatic resection. He showed leukocytosis of 25,450/microL along with elevated serum G-CSF. Histological examination of surgical samples demonstrated immunohistochemical staining for G-CSF, but not for G-CSF receptor. The patient survived without recurrence for four years, but ultimately passed away with multiple bone metastases. In light of the above, clinicians may consider G-CSF-producing HCC when encountering patients with leukocytosis and a hepatic tumor. More cases are needed to clarify the clinical picture of G-CSF-producing HCC.
MeSH Terms
-
Aged
Bone Neoplasms/secondary
Carcinoma, Hepatocellular/*metabolism/pathology
Fatal Outcome
Granulocyte Colony-Stimulating Factor/*metabolism
Humans
Liver Neoplasms/*metabolism/pathology
Male
Receptors, Granulocyte Colony-Stimulating Factor/metabolism
Receptors, Granulocyte Colony-Stimulating Factor
Granulocyte Colony-Stimulating Factor
Figure
Reference
-
1. Bradley TR, Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966. 44:287–299.2. Robinson WA. Granulocytosis in neoplasia. Ann N Y Acad Sci. 1974. 230:212–218.
Article3. Asano S, Urabe A, Okabe T, Sato N, Kondo Y. Demonstration of granulopoietic factor(s) in the plasma of nude mice transplanted with a human lung cancer and in the tumor tissue. Blood. 1977. 49:845–852.
Article4. Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (1). N Engl J Med. 1992. 327:28–35.5. Yamamoto S, Takashima S, Ogawa H, Kuroda T, Yamamoto M, Takeda A, Nakmaura H. Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol. 1999. 34:640–644.
Article6. Araki K, Kishihara F, Takahashi K, Matsumata T, Shimura T, Suehiro T, Kuwano H. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007. 27:716–721.
Article7. Shimamura K, Fujimoto J, Hata J, Akatsuka A, Ueyama Y, Watanabe T, Tamaoki N. Establishment of specific monoclonal antibodies against recombinant human granulocyte colony-stimulating factor (hG-CSF) and their application for immunoperoxidase staining of paraffin-embedded sections. J Histochem Cytochem. 1990. 38:283–286.
Article8. Higaki I, Hirohashi K, Fukushima S, Wanibuchi H, Seike N, Yamane T, Kubo S, Tanaka H, Shuto T, Yamamoto T, Kinoshita H. Renal pelvic carcinoma producing granulocyte colony-stimulating factor: report of a case. Surg Today. 2001. 31:266–268.
Article9. Segawa K, Ueno Y, Kataoka T. In vivo tumor growth enhancement by granulocyte colony-stimulating factor. Jpn J Cancer Res. 1991. 82:440–447.
Article10. Tachibana M, Miyakawa A, Tazaki H, Nakamura K, Kubo A, Hata J, Nishi T, Amano Y. Autocrine growth of transitional cell carcinoma of the bladder induced by granulocyte-colony stimulating factor. Cancer Res. 1995. 55:3438–3443.11. Baba M, Hasegawa H, Nakayabu M, Shimizu N, Suzuki S, Kamada N, Tani K. Establishment and characteristics of a gastric cancer cell line (HuGC-OOHIRA) producing high levels of G-CSF, GM-CSF, and IL-6: the presence of autocrine growth control by G-CSF. Am J Hematol. 1995. 49:207–215.
Article12. Kyo S, Kanaya T, Takakura M, Inoue M. A case of cervical cancer with aggressive tumor growth: possible autocrine growth stimulation by G-CSF and Il-6. Gynecol Oncol. 2000. 78:383–387.
Article13. Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999. 155:1557–1567.
Article14. Tsuzuki H, Fujieda S, Sunaga H, Noda I, Saito H. Expression of granulocyte colony-stimulating factor receptor correlates with prognosis in oral and mesopharyngeal carcinoma. Cancer Res. 1998. 58:794–800.15. Suzuki A, Takahashi T, Okuno Y, Tsuyuoka R, Fukumoto M, Nakamura K, Imura H. IL-1 production as a regulator of G-CSF and IL-6 production in CSF-producing cell lines. Br J Cancer. 1992. 65:515–518.
Article16. Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999. 179:Suppl 2. S294–S304.
Article17. Luheshi GN. Cytokines and fever. Mechanisms and sites of action. Ann N Y Acad Sci. 1998. 856:83–89.
Article18. Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989. 242:237–239.
Article19. Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990. 12:1179–1186.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- A Metastatic Granulocyte Colony-Stimulating Factor Producing Sarcomatoid Carcinoma of the Lung Causing Jejunal Intussusception: Report of a Case