J Korean Med Sci.
2011 Apr;26(4):587-591. 10.3346/jkms.2011.26.4.587.
Carrier Woman of Duchenne Muscular Dystrophy Mimicking Inflammatory Myositis
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Inha University Hospital, Incheon, Korea. parkwon@inha.ac.kr
- 2Department of Pathology, College of Medicine, Yonsei University, Seoul, Korea.
- 3Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1777894
- DOI: http://doi.org/10.3346/jkms.2011.26.4.587
Abstract
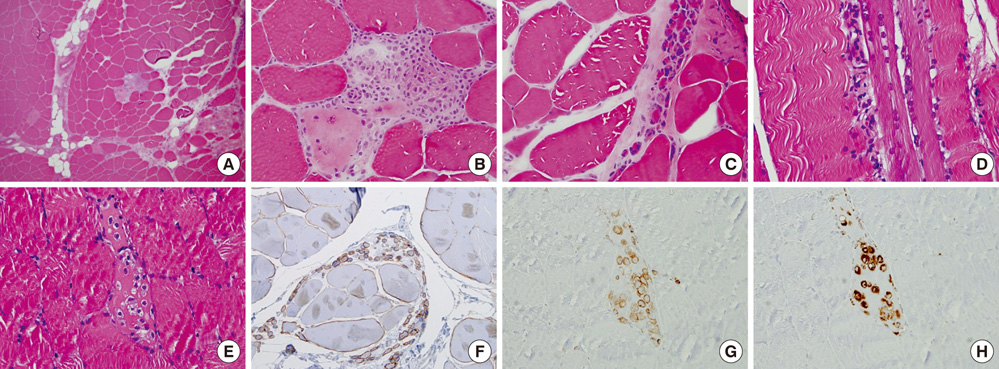
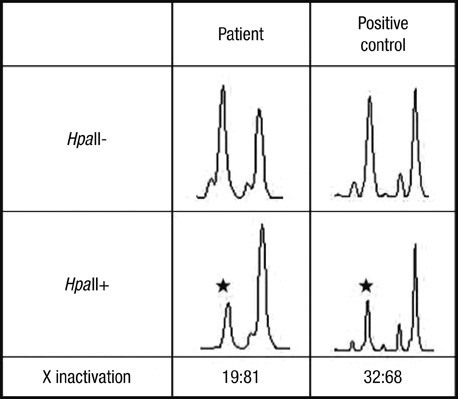
- Carrier woman of Duchenne muscular dystrophy (DMD) can mimic the inflammatory myositis in presenting symptoms. Two diseases should be differentiated by the clinical history, muscle biopsy and genetic study. There are few reports in which both histochemical and genetic study showed the possible link of overlapping inflammatory pathophysiology with dystrophinopathy. We report a 40-yr-old woman who presented with subacute proximal muscle weakness and high serum level of creatine kinase. She had a history of Graves' disease and fluctuation of serum liver aminotransferase without definite cause. MRI, EMG and NCV were compatible with proximal muscle myopathy. Muscle biopsy on vastus lateralis showed suspicious perifascicular atrophy and infiltration of mono-macrophage lineage cells complicating the diagnosis. Dystrophin staining showed heterogeneous diverse findings from normal to interrupted mosaic pattern. Multiple ligation probe amplification and X chromosome inactivation test confirmed DMD gene deletion mutation in exon 44 and highly skewed X inactivation.
MeSH Terms
Figure
Cited by 1 articles
-
Clinical and Genetic Characterization of Female Dystrophinopathy
Seung Ha Lee, Jung Hwan Lee, Kyung-A Lee, Young-Chul Choi
J Clin Neurol. 2015;11(3):248-251. doi: 10.3988/jcn.2015.11.3.248.
Reference
-
1. Roberts RG. Dystrophin, its gene, and the dystrophinopathies. Adv Genet. 1995. 33:177–231.2. Hoogerwaard EM, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, Leschot NJ, Van Essen AJ, Brunner HG, van der Wouw PA, Wilde AA, de Visser M. Signs and symptoms of Duchenne muscular dystrophy and Becker muscular dystrophy among carriers in The Netherlands: a cohort study. Lancet. 1999. 353:2116–2119.3. Gatta V, Scarciolla O, Gaspari AR, Palka C, De Angelis MV, Di Muzio A, Guanciali-Franchi P, Calabrese G, Uncini A, Stuppia L. Identification of deletions and duplications of the DMD gene in affected males and carrier females by multiple ligation probe amplification (MLPA). Hum Genet. 2005. 117:92–98.4. Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet. 1992. 51:1229–1239.5. Xinhua Bao, Shengling Jiang, Fuying Song, Hong Pan, Meirong Li, Wu XR. X chromosome inactivation in Rett Syndrome and its correlations with MECP2 mutations and phenotype. J Child Neurol. 2008. 23:22–25.6. Lee KA, Han SH, Choi JR, Chung JS, Choi YC. Becker muscular dystrophy with r(X) carrying an out-of-frame DMD deletion. Pediatr Neurol. 2008. 39:129–132.7. Yang MS, Lee DK, Oh KY, Choi KS, Lee KH. Duchenne muscular dystrophy in a girl with Turner syndrome. J Korean Acad Rehabil Med. 2005. 29:537–540.8. Seo CD, Lee YJ, Lee EH, Jeong MH, Yum MS, Ko JM, Yoo HW, Ko TS. The clinical features, immunostaining and genetic study in Duchenne/Becker muscular dystrophy. J Korean Child Neurol Soc. 2009. 17:40–49.9. Emery AE. The muscular dystrophies. Lancet. 2002. 359:687–695.10. Bonilla E, Schmidt B, Samitt CE, Miranda AF, Hays AP, de Oliveira AB, Chang HW, Servidei S, Ricci E, Younger DS, Dimauro S. Normal and dystrophin-deficient muscle fibers in carriers of the gene for Duchenne muscular dystrophy. Am J Pathol. 1988. 133:440–445.11. Yoshioka M, Yorifuji T, Mituyoshi I. Skewed X inactivation in manifesting carriers of Duchenne muscular dystrophy. Clin Genet. 1998. 53:102–107.12. Norman A, Harper P. A survey of manifesting carriers of Duchenne and Becker muscular dystrophy in Wales. Clin Genet. 1989. 36:31–37.13. Hoogerwaard EM, van der Wouw PA, Wilde AA, Bakker E, Ippel PF, Oosterwijk JC, Majoor-Krakauer DF, van Essen AJ, Leschot NJ, de Visser M. Cardiac involvement in carriers of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 1999. 9:347–351.14. Hoogerwaard EM, Ginjaar IB, Bakker E, de Visser M. Dystrophin analysis in carriers of Duchenne and Becker muscular dystrophy. Neurology. 2005. 65:1984–1986.15. McDouall RM, Dunn MJ, Dubowitz V. Nature of the mononuclear infiltrate and the mechanism of muscle damage in juvenile dermatomyositis and Duchenne muscular dystrophy. J Neurol Sci. 1990. 99:199–217.16. Nirmalananthan N, Holton JL, Hanna MG. Is it really myositis? A consideration of the differential diagnosis. Curr Opin Rheumatol. 2004. 16:684–691.17. Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003. 2:731–740.18. Deconinck N, Dan B. Pathophysiology of duchenne muscular dystrophy: current hypotheses. Pediatr Neurol. 2007. 36:1–7.19. Dalakas MC. Polymyositis, dermatomyositis and inclusion-body myositis. N Engl J Med. 1991. 325:1487–1498.20. Radley HG, De Luca A, Lynch GS, Grounds MD. Duchenne muscular dystrophy: focus on pharmaceutical and nutritional interventions. Int J Biochem Cell Biol. 2007. 39:469–477.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carrier detection of duchenne muscular dystrophy with closelylinked RFLPs.
- A Paucisymptomatic HyperCKemia Patient Diagnosed with Manifesting Female Duchenne Muscular Dystrophy Carrier
- A clinical study on Duchenne muscular dystrophy
- Duchenne Muscular Dystrophy Complicated With Dilated Cardiomyopathy and Cerebral Infarction
- A Clinical Study on Duchenne Muscular Dystrophy in Childhood