Korean J Radiol.
2008 Feb;9(1):29-37. 10.3348/kjr.2008.9.1.29.
Sonography Guided Percutaneous Radiofrequency Ablation of Hepatocellular Carcinoma: Effect of Cooperative Training on the Pretreatment Assessment of the Operation's Feasibility
- Affiliations
-
- 1Department of Radiology, Korea University Anam Hospital, Seoul, Korea.
- 2Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. hklim@smc.samsung.co.kr
- 3Biostatistics Unit, Samsung Biomedical Research Institute, Seoul, Korea.
- KMID: 1734272
- DOI: http://doi.org/10.3348/kjr.2008.9.1.29
Abstract
OBJECTIVE
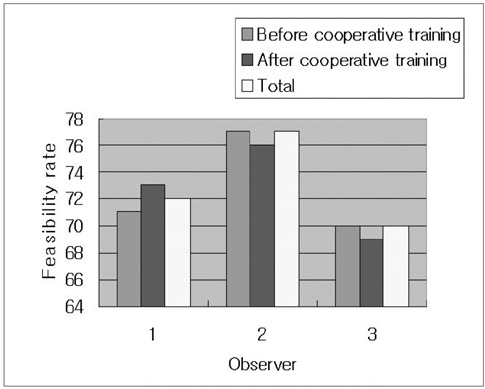
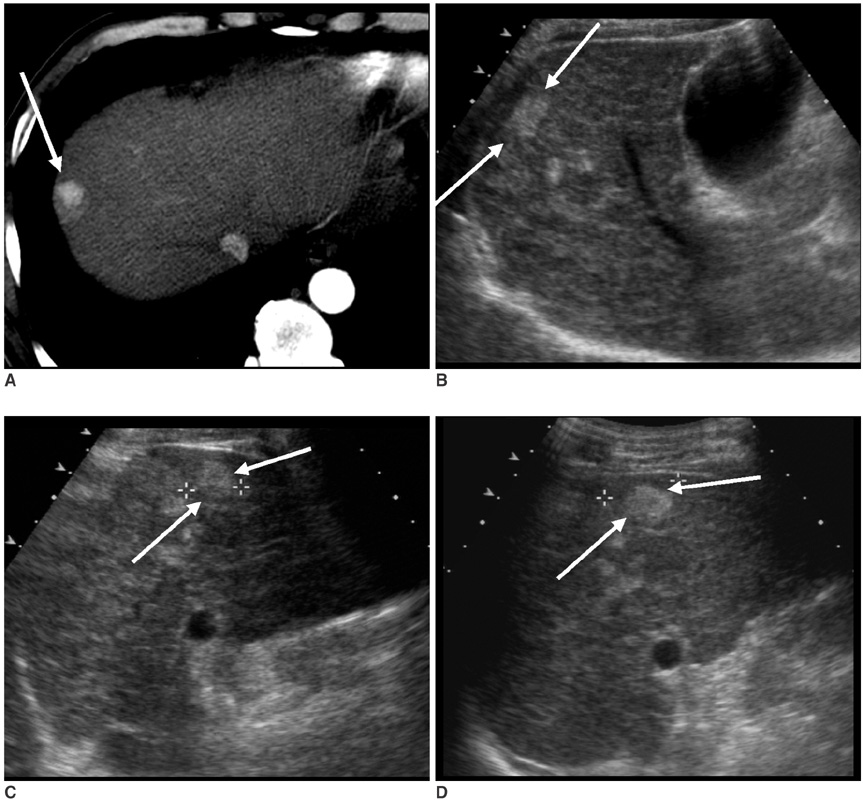
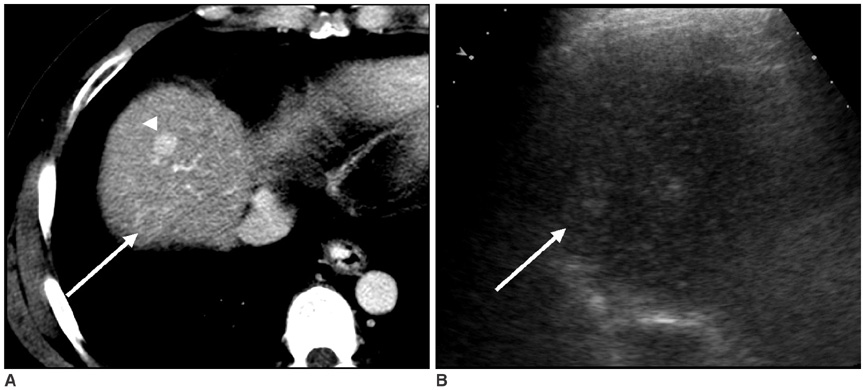
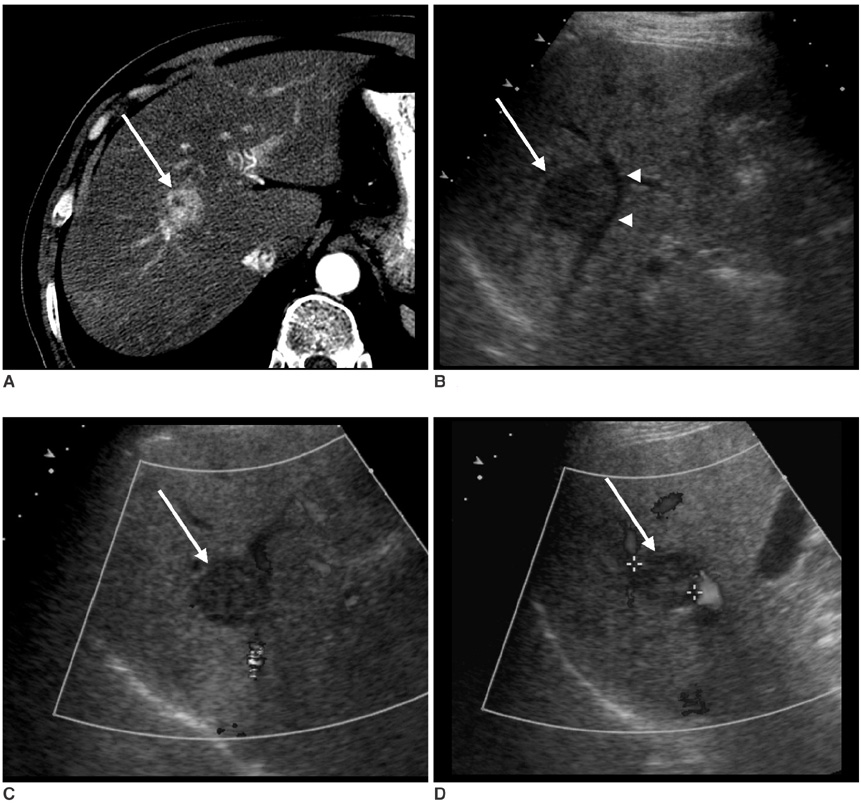
The aim of this study is to investigate the effects of cooperative training on the pretreatment assessment of the feasibility to perform Ultrasonography (US) guided percutaneous radiofrequency ablation for patients afflicted with hepatocellular carcinoma. MATERIALS AND METHODS: In our prospective study, 146 patients with 200 hepatocellular carcinomas were referred for radiofrequency ablation after triage by hepatologists. Three radiologists with different levels of experience performed the planning US before (group I) and after (group II) cooperative training, to evaluate whether radiofrequency ablation was feasible. The feasibility rates considered eligible according to our criteria were evaluated. In addition, we analyzed the reasons for the lack of feasibility were analyzed. The interobserver agreement for the assessment of feasibility before and after training was also calculated. RESULTS: The overall feasibility rates for both groups was 73%. No significant difference in the feasibility rates was observed. The feasibility rates of each observer for group I were 71% (observer 1), 77% (observer 2) and 70% (observer 3) and those for group II were 73%, 76% and 69%, respectively. In the tumors (n = 164) considered ineligible, the two most common causes for refraining from performing radiofrequency ablation included non-visualization of the tumor (62%) and the absence of a safe route for the percutaneous approach (38%). We found moderate interobserver agreement for all observers before cooperative training and a good agreement after training. CONCLUSION: Although the cooperative training did not affect the feasibility rate of each observer, it improved the interobserver agreement for assessing the feasibility of performing US guided radiofrequency ablation, which may reduce unnecessary admission or delayed treatment.
MeSH Terms
Figure
Reference
-
1. Ahmed M, Goldberg SN. Thermal ablation therapy for hepatocellular carcinoma. J Vasc Interv Radiol. 2002. 13:S231–S244.2. Dodd GD 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000. 20:9–27.3. Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities-part II. J Vasc Interv Radiol. 2001. 12:1135–1148.4. Goldberg SN, Charboneau JW, Dodd GD 3rd, Dupuy DE, Gervais DA, Gillams AR, et al. Image-guided tumor ablation: proposal for standardization of terms and reporting criteria. Radiology. 2003. 228:335–345.5. Honda N, Guo Q, Uchida H, Ohishi H, Hiasa Y. Percutaneous hot saline injection therapy for hepatic tumors: an alternative to percutaneous ethanol injection therapy. Radiology. 1994. 190:53–57.6. Livraghi T, Giorgio A, Marin G, Salmi A, de Sio I, Bolondi L, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995. 197:101–108.7. Vogl TJ, Muller PK, Hammerstingl R, Weinhold N, Mack MG, Philipp C, et al. Malignant liver tumors treated with MR imaging-guided laser-induced thermotherapy: technique and prospective results. Radiology. 1995. 196:257–265.8. Lencioni R, Cioni D, Crocetti L, Bartolozzi C. Percutaneous ablation of hepatocellular carcinoma: state-of-the-art. Liver Transpl. 2004. 10:S91–S97.9. Goldberg SN. Comparison of techniques for image-guided ablation of focal liver tumors. Radiology. 2002. 223:304–307.10. Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000. 88:2452–2463.11. Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000. 174:323–331.12. Lencioni R, Cioni D, Bartolozzi C. Percutaneous radiofrequency thermal ablation of liver malignancies: techniques, indications, imaging findings, and clinical results. Abdom Imaging. 2001. 26:345–360.13. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001. 176:3–16.14. Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. Tumor ablation with radio-frequency energy. Radiology. 2000. 217:633–646.15. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.16. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999. 210:655–661.17. Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000. 217:119–126.18. Gazelle GS, Haaga JR. Guided percutaneous biopsy of intraabdominal lesions. AJR Am J Roentgenol. 1989. 153:929–935.19. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST, et al. Learning curve for radiofrequency ablation of liver tumors: prospective analysis of initial 100 patients in a tertiary institution. Ann Surg. 2004. 239:441–449.20. Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001. 35:421–430.21. Agresti A. An introduction to categorical data analysis. 1996. New York: Wiley.22. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977. 33:159–174.23. Lim HK. Radiofrequency thermal ablation of hepatocellular carcinomas. Korean J Radiol. 2000. 1:175–184.24. Jolesz FA. Interventional magnetic resonance imaging, computed tomography, and ultrasound. Acad Radiol. 1995. 2:Suppl 2. S124–S125.25. Choi D, Lim HK, Lee WJ, Kim SH, Kim MJ, Kim SK, et al. Radiofrequency ablation of liver cancer: early evaluation of therapeutic response with contrast-enhanced ultrasonography. Korean J Radiol. 2004. 5:185–198.26. Danford DA, Kugler JD, Deal B, Case C, Friedman RA, Saul JP, et al. The learning curve for radiofrequency ablation of tachyarrhythmias in pediatric patients. Participating members of the Pediatric Electrophysiology Society. Am J Cardiol. 1995. 75:587–590.27. Couinaud C. Le Foie: Etudes anatomiques et chirurgicales. 1957. Paris: Masson.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Small Bowel Perforation after Percutaneous Ultrasound-guided Radiofrequency Ablation of Hepatocellular Carcinoma
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Microwave thermosphere versus radiofrequency ablation for hepatocellular carcinoma: Are we approaching the time to end the debate?
- Percutaneous cryoablation for hepatocellular carcinoma
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?