J Vet Sci.
2008 Sep;9(3):233-240. 10.4142/jvs.2008.9.3.233.
Immunohistochemical identification and quantitative analysis of cytoplasmic Cu/Zn superoxide dismutase in mouse organogenesis
- Affiliations
-
- 1College of Veterinary Medicine and Research Institute of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. synam@cbu.ac.kr
- 2CKD Research Institute, Chong Kun Dang Pharm., Chonan P.O. Box 74, Chonan 330-600, Korea.
- KMID: 1718575
- DOI: http://doi.org/10.4142/jvs.2008.9.3.233
Abstract
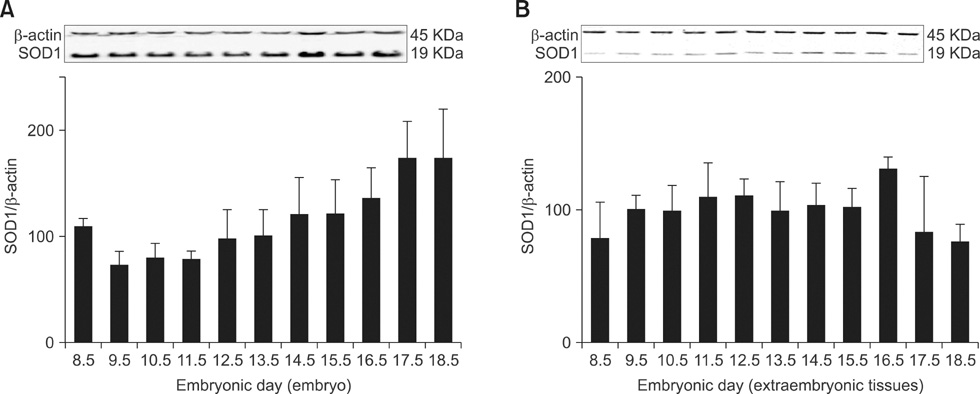
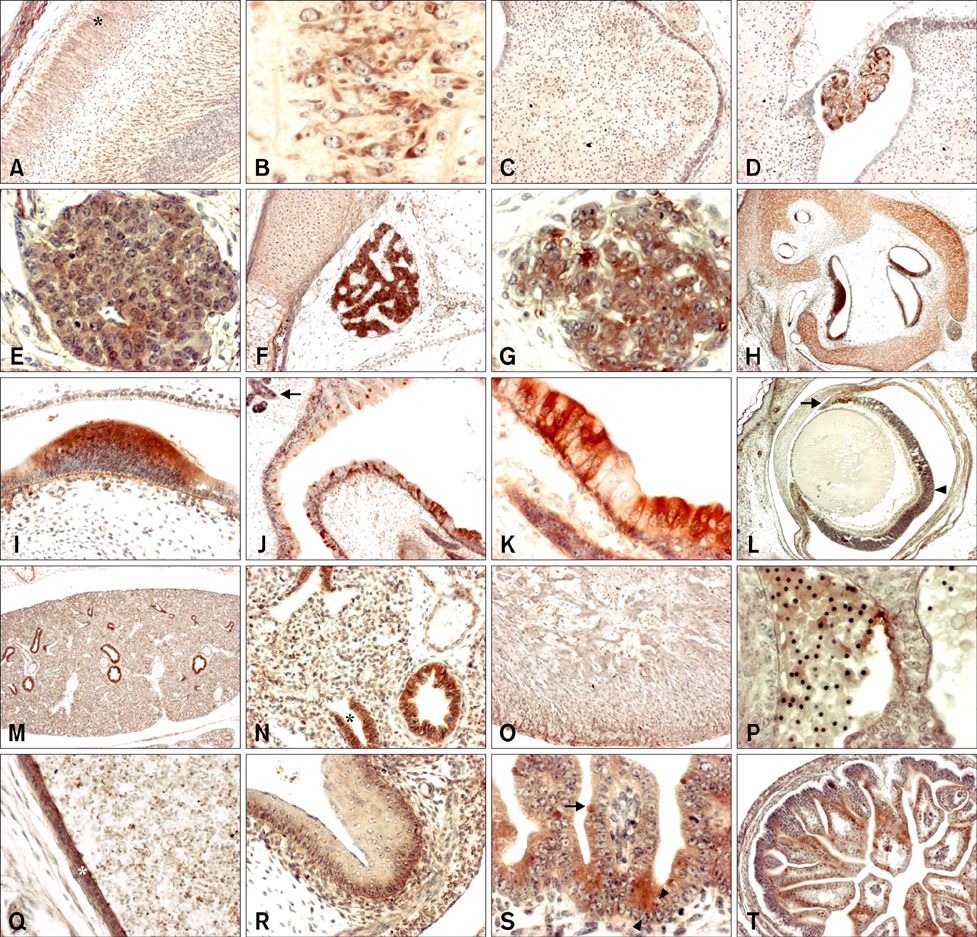
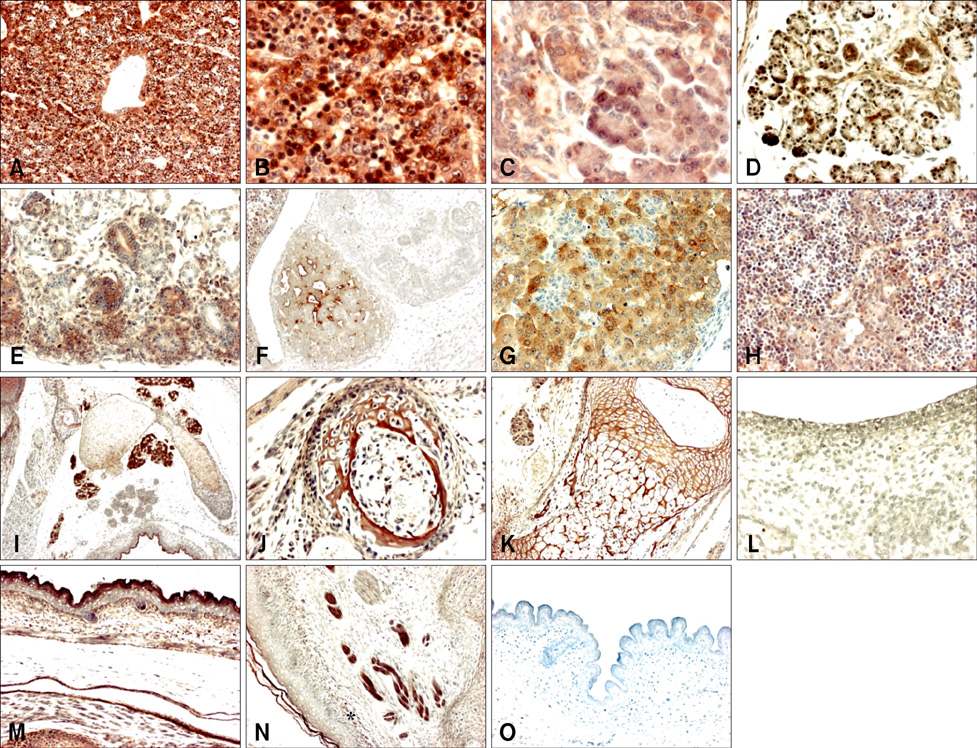
- Cytoplasmic Cu/Zn superoxide dismutase (SOD1) is an antioxidant enzyme that converts superoxide to hydrogen peroxide in cells. Its spatial distribution matches that of superoxide production, allowing it to protect cells from oxidative stress. SOD1 deficiencies result in embryonic lethality and a wide range of pathologies in mice, but little is known about normal SOD1 protein expression in developing embryos. In this study, the expression pattern of SOD1 was investigated in post-implantation mouse embryos and extraembryonic tissues, including placenta, using Western blotting and immunohistochemical analyses. SOD1 was detected in embryos and extraembryonic tissues from embryonic day (ED) 8.5 to 18.5. The signal in embryos was observed at the lowest level on ED 9.5-11.5, and the highest level on ED 17.5-18.5, while levels remained constant in the surrounding extraembryonic tissues during all developmental stages examined. Immunohistochemical analysis of SOD1 expression on ED 13.5-18.5 revealed its ubiquitous distribution throughout developing organs. In particular, high levels of SOD1 expression were observed in the ependymal epithelium of the choroid plexus, ganglia, sensory cells of the olfactory and vestibulocochlear epithelia, blood cells and vessels, hepatocytes and hematopoietic cells of the liver, lymph nodes, osteogenic tissues, and skin. Thus, SOD1 is highly expressed at late stages of embryonic development in a cell- and tissue-specific manner, and can function as an important antioxidant enzyme during organogenesis in mouse embryos.
MeSH Terms
Figure
Reference
-
1. Barkats M, Horellou P, Colin P, Millecamps S, Faucon-Biguet N, Mallet J. 1-Methyl-4-phenylpyridinium neurotoxicity is attenuated by adenoviral gene transfer of human Cu/Zn superoxide dismutase. J Neurosci Res. 2006. 83:233–242.
Article2. Chen EY, Fujinaga M, Giaccia AJ. Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology. 1999. 60:215–225.
Article3. Chen SY, Sulik KK. Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol Clin Exp Res. 1996. 20:1071–1076.
Article4. Crapo JD, Oury T, Rabouille C, Slot JW, Chang LY. Copper,zinc superoxide dismutase is primarily a cytosolic protein in human cells. Proc Natl Acad Sci USA. 1992. 89:10405–10409.
Article5. Cuzzocrea S, Mazzon E, Paola RD, Genovese T, Muià C, Caputi AP, Salvemini D. Effects of combination M40403 and dexamethasone therapy on joint disease in a rat model of collagen-induced arthritis. Arthritis Rheum. 2005. 52:1929–1940.
Article6. Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007. 81:155–162.
Article7. Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002. 91:938–944.
Article8. Elchuri S, Oberley TD, Qi W, Eisenstein RS, Jackson Roberts L, Van Remmen H, Epstein CJ, Huang TT. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005. 24:367–380.
Article9. Frederiks WM, Bosch KS. Localization of superoxide dismutase activity in rat tissues. Free Radic Biol Med. 1997. 22:241–248.
Article10. Hanson LA. Session 1: Feeding and infant development breast-feeding and immune function. Proc Nutr Soc. 2007. 66:384–396.
Article11. Ho YS, Gargano M, Cao J, Bronson RT, Heimler I, Hutz RJ. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998. 273:7765–7769.
Article12. Iuchi Y, Okada F, Onuma K, Onoda T, Asao H, Kobayashi M, Fujii J. Elevated oxidative stress in erythrocytes due to a SOD1 deficiency causes anemia and triggers autoantibody production. Biochem J. 2007. 402:219–227.
Article13. Keithley EM, Canto C, Zheng QY, Wang X, Fischel-Ghodsian N, Johnson KR. Cu/Zn superoxide dismutase and age-related hearing loss. Hear Res. 2005. 209:76–85.
Article14. Khan JY, Black SM. Developmental changes in murine brain antioxidant enzymes. Pediatr Res. 2003. 54:77–82.
Article15. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969. 244:6049–6055.16. McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999. 20:1–8.
Article17. Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med. 2006. 40:1993–2004.
Article18. Munim A, Asayama K, Dobashi K, Suzuki K, Kawaoi A, Kato K. Immunohistochemical localization of superoxide dismutases in fetal and neonatal rat tissues. J Histochem Cytochem. 1992. 40:1705–1713.
Article19. Ogawa T, Ohira A, Amemiya T. Manganese and copper-zinc superoxide dismutases in the developing rat retina. Acta Histochem. 1997. 99:1–12.
Article20. Okado-Matsumoto A, Fridovich I. Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem. 2001. 276:38388–38393.21. Pardo CA, Xu Z, Borchelt DR, Price DL, Sisodia SS, Cleveland DW. Superoxide dismutase is an abundant component in cell bodies, dendrites, and axons of motor neurons and in a subset of other neurons. Proc Natl Acad Sci USA. 1995. 92:954–958.
Article22. Pérez MJ, Cederbaum AI. Adenovirus-mediated expression of Cu/Zn- or Mn-superoxide dismutase protects against CYP2E1-dependent toxicity. Hepatology. 2003. 38:1146–1158.
Article23. Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol. 2001. 6:117–123.
Article24. Shaw PJ, Ince PG, Falkous G, Mantle D. Oxidative damage to protein in sporadic motor neuron disease spinal cord. Ann Neurol. 1995. 38:691–695.
Article25. Sheppard FR, Kelher MR, Moore EE, McLaughlin NJ, Banerjee A, Silliman CC. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005. 78:1025–1042.
Article26. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007. 39:44–84.
Article27. Wang Y, Walsh SW. Increased superoxide generation is associated with decreased superoxide dismutase activity and mRNA expression in placental trophoblast cells in preeclampsia. Placenta. 2001. 22:206–212.
Article28. Weisiger RA, Fridovich I. Mitochondrial superoxide simutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973. 248:4793–4796.29. Wentzel P, Eriksson UJ. Antioxidants diminish developmental damage induced by high glucose and cyclooxygenase inhibitors in rat embryos in vitro. Diabetes. 1998. 47:677–684.
Article30. Yon JM, Baek IJ, Lee SR, Jin Y, Kim MR, Nahm SS, Kim JS, Ahn B, Lee BJ, Yun YW, Nam SY. The spatio-temporal expression pattern of cytoplasmic Cu/Zn superoxide dismutase (SOD1) mRNA during mouse embryogenesis. J Mol Histol. 2008. 39:95–103.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Purification and characterization of a Cu, Zn-superoxide dismutase from adult Paragonimus westermani
- Study on Superoxide Dismutase Activity in Psoriatic Skin
- The change of superoxide dismutase activity in mouse skin by ultraviolet radiation
- Differential Regulation of Antioxidant Enzymes during Monocyte Differentiation
- Role of Inducibility of Superoxide Dismutases and Metallothionein of Mouse Lungs by Paraquat in Aging