Korean J Radiol.
2013 Jun;14(3):416-422. 10.3348/kjr.2013.14.3.416.
Magnetic Field Interactions of Copper-Containing Intrauterine Devices in 3.0-Tesla Magnetic Resonance Imaging: In Vivo Study
- Affiliations
-
- 1Department of Radiology, Medical University of Vienna, Vienna 1090, Austria. vanessa.berger-kulemann@meduniwien.ac.at
- 2Department of Obstetrics and Gynecology, Medical University of Vienna, Vienna 1090, Austria.
- KMID: 1705453
- DOI: http://doi.org/10.3348/kjr.2013.14.3.416
Abstract
OBJECTIVE
An ex vivo study found a copper-containing intrauterine device (IUD) to be safe for women undergoing an MRI examination at a 3.0-T field. No significant artifacts caused by the metallic implant were detected. However, there are still no in vivo data about these concerns. The aim of this study was to evaluate 3.0-T magnetic field interactions of copper-containing IUDs in vivo.
MATERIALS AND METHODS
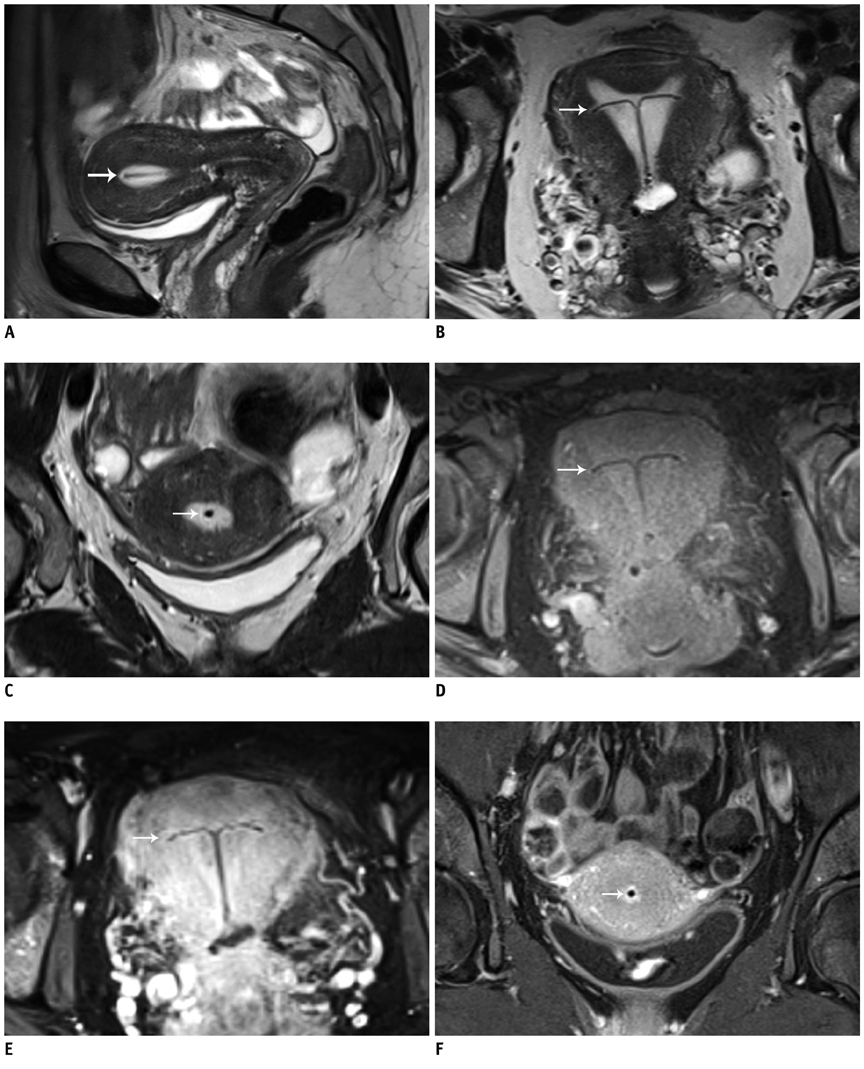
Magnetic field interactions and potential adverse events were evaluated in 33 women using a questionnaire-based telephone survey. Two experienced radiologists performed artifact evaluation on MR images of the pelvis.
RESULTS
Eighteen patients were eligible for the survey. One patient reported a dislocation of the IUD after the MR examination. All other patients had no signs of field interactions. No IUD-related artifacts were found.
CONCLUSION
MRI at 3.0-T is possible for women with copper-containing IUDs. However, consulting a gynecologist to check the correct position of the IUD and exclude complications after an MR examination is highly recommended. High-quality clinical imaging of the female pelvis can be performed without a loss in image quality.
Keyword
MeSH Terms
Figure
Reference
-
1. Peipert JF, Zhao Q, Allsworth JE, Petrosky E, Madden T, Eisenberg D, et al. Continuation and satisfaction of reversible contraception. Obstet Gynecol. 2011. 117:1105–1113.2. de Araujo FF, Barbieri M, Guazzelli CA, Lindsey PC. The T 380A intrauterine device: a retrospective 5-year evaluation. Contraception. 2008. 78:474–478.3. Thonneau PF, Almont T. Contraceptive efficacy of intrauterine devices. Am J Obstet Gynecol. 2008. 198:248–253.4. Farr G, Amatya R, Doh A, Ekwempu CC, Toppozada M, Ruminjo J. An evaluation of the copper-T 380A IUD's safety and efficacy at three African centers. Contraception. 1996. 53:293–298.5. Reinprayoon D, Gilmore C, Farr G, Amatya R. Twelve-month comparative multicenter study of the TCu 380A and ML 250 intrauterine devices in Bangkok, Thailand. Contraception. 1998. 58:201–206.6. Suri V, Aggarwal N, Kaur R, Chaudhary N, Ray P, Grover A. Safety of intrauterine contraceptive device (copper T 200 B) in women with cardiac disease. Contraception. 2008. 78:315–318.7. Schwarz EB, Hess R, Trussell J. Contraception for cancer survivors. J Gen Intern Med. 2009. 24:Suppl 2. S401–S406.8. Peri N, Graham D, Levine D. Imaging of intrauterine contraceptive devices. J Ultrasound Med. 2007. 26:1389–1401.9. Craven I, Griffiths PD, Hoggard N. Magnetic resonance imaging of epilepsy at 3 Tesla. Clin Radiol. 2011. 66:278–286.10. Stankiewicz JM, Glanz BI, Healy BC, Arora A, Neema M, Benedict RH, et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. 2011. 21:e50–e56.11. Arizono S, Isoda H, Maetani YS, Hirokawa Y, Shimada K, Nakamoto Y, et al. High spatial resolution 3D MR cholangiography with high sampling efficiency technique (SPACE): comparison of 3T vs. 1.5T. Eur J Radiol. 2010. 73:114–118.12. Kataoka M, Kido A, Koyama T, Isoda H, Umeoka S, Tamai K, et al. MRI of the female pelvis at 3T compared to 1.5T: evaluation on high-resolution T2-weighted and HASTE images. J Magn Reson Imaging. 2007. 25:527–534.13. Michaely HJ, Attenberger UI, Kramer H, Nael K, Reiser MF, Schoenberg SO. Abdominal and pelvic MR angiography. Magn Reson Imaging Clin N Am. 2007. 15:301–314. v–vi.14. Bauer JS, Monetti R, Krug R, Matsuura M, Mueller D, Eckstein F, et al. Advances of 3T MR imaging in visualizing trabecular bone structure of the calcaneus are partially SNR-independent: analysis using simulated noise in relation to micro-CT, 1.5T MRI, and biomechanical strength. J Magn Reson Imaging. 2009. 29:132–140.15. Pasquale SA, Russer TJ, Foldesy R, Mezrich RS. Lack of interaction between magnetic resonance imaging and the copper-T380A IUD. Contraception. 1997. 55:169–173.16. Hess T, Stepanow B, Knopp MV. Safety of intrauterine contraceptive devices during MR imaging. Eur Radiol. 1996. 6:66–68.17. Kido A, Togashi K, Kataoka ML, Nakai A, Koyama T, Fujii S. Intrauterine devices and uterine peristalsis: evaluation with MRI. Magn Reson Imaging. 2008. 26:54–58.18. Mühler M, Taupitz M. [How safe is magnetic resonance imaging in patients with contraceptive implants?]. Radiologe. 2006. 46:574–578.19. Zieman M, Kanal E. Copper T 380A IUD and magnetic resonance imaging. Contraception. 2007. 75:93–95.20. Shellock FG. MRIsafety.com. Accessed August 29, 2012. http://www.mrisafety.com/list.asp/.21. O'Brien PA, Kulier R, Helmerhorst FM, Usher-Patel M, d'Arcangues C. Copper-containing, framed intrauterine devices for contraception: a systematic review of randomized controlled trials. Contraception. 2008. 77:318–327.22. Rasheed SM, Abdelmonem AM. Complications among adolescents using copper intrauterine contraceptive devices. Int J Gynaecol Obstet. 2011. 115:269–272.23. Glass T, Baker T, Kauffman RP. Migration of an intrauterine contraceptive device during the course of pregnancy: a case report. J Minim Invasive Gynecol. 2009. 16:81–83.24. Andersson K, Ryde-Blomqvist E, Lindell K, Odlind V, Milsom I. Perforations with intrauterine devices. Report from a Swedish survey. Contraception. 1998. 57:251–255.25. Markovitch O, Klein Z, Gidoni Y, Holzinger M, Beyth Y. Extrauterine mislocated IUD: is surgical removal mandatory? Contraception. 2002. 66:105–108.26. Turok DK, Gurtcheff SE, Gibson K, Handley E, Simonsen S, Murphy PA. Operative management of intrauterine device complications: a case series report. Contraception. 2010. 82:354–357.27. Taras AR, Kaufman JA. Laparoscopic retrieval of intrauterine device perforating the sigmoid colon. JSLS. 2010. 14:453–455.28. Caliskan E, Oztürk N, Dilbaz BO, Dilbaz S. Analysis of risk factors associated with uterine perforation by intrauterine devices. Eur J Contracept Reprod Health Care. 2003. 8:150–155.29. Harrison-Woolrych M, Ashton J, Coulter D. Uterine perforation on intrauterine device insertion: is the incidence higher than previously reported? Contraception. 2003. 67:53–56.30. MagResource - MRI safety worldwide. Accessed August 29, 2012. http://www.magresource.com/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High field strength magnetic resonance imaging of cardiovascular diseases
- Introduction to high field strength magnetic resonance imaging
- The current status of three-dimensional ultrasonography in gynaecology
- Efforts of the Past 20 Years for Proved Magnetic Resonance Imaging Safety of Medtronic Implantable Cardiac Devices
- Cardiac Magnetic Resonance with a Conditional Pacemaker at Three Tesla Field Strength