Korean J Ophthalmol.
2013 Aug;27(4):235-242. 10.3341/kjo.2013.27.4.235.
The Effect of Bevacizumab versus Ranibizumab in the Treatment of Corneal Neovascularization: A Preliminary Study
- Affiliations
-
- 1Department of Ophthalmology, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea. eyedr0823@hotmail.com
- KMID: 1705418
- DOI: http://doi.org/10.3341/kjo.2013.27.4.235
Abstract
- PURPOSE
To compare the short term effects of bevacizumab and ranibizumab injections on the regression of corneal neovascularization (NV).
METHODS
Sixteen eyes of 16 patients with corneal NV were randomly assigned for an injection with 2.5 mg of bevacizumab (group 1, n = 8) or 1 mg of ranibizumab (group 2, n = 8) through subconjunctival and intrastromal routes. The patients were prospectively followed-up for one month after the injections. Corneal NV areas, as shown on corneal slit-lamp photographs stored in JPEG format, were calculated using Image J software before the injection, one week after the injection, and one month after the injection. The corneal NV areas were compared before and after the injections.
RESULTS
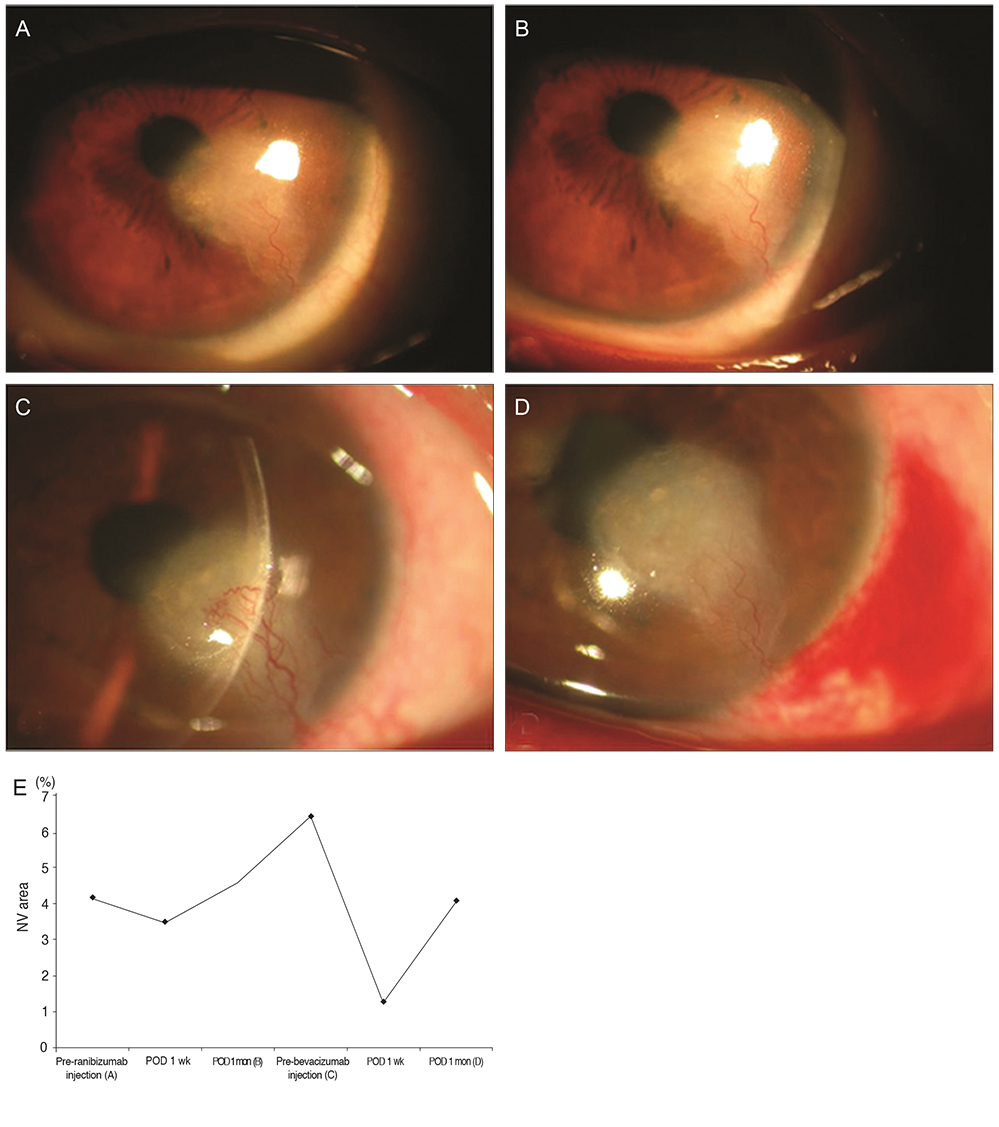
Seven women and nine men, with an average age of 51 years, presented with corneal NV secondary to herpetic keratitis (7 cases), graft rejection (6), chemical burn (1), pemphigoid (1), and recurrent ulcer (1). In group I, the preoperative corneal NV area (8.75 +/- 4.33%) was significantly decreased to 5.62 +/- 3.86% one week after the injection and to 6.35 +/- 3.02% one month after the injection (p = 0.012, 0.012, respectively). The corneal NV area in group 2 also exhibited a significant change, from 7.37 +/- 4.33% to 6.72 +/- 4.16% one week after the injection (p = 0.012). However, no significant change was observed one month after the injection. The mean decrease in corneal NV area one month after injection in group 1 (28.4 +/- 9.01%) was significantly higher than in group 2 (4.51 +/- 11.64%, p = 0.001).
CONCLUSIONS
Bevacizumab injection resulted in a more effective and stable regression of corneal NV compared to the ranibizumab injection. The potency and dose of these two drugs for the regression of corneal NV require further investigation.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Angiogenesis Inhibitors/*therapeutic use
Antibodies, Monoclonal, Humanized/*therapeutic use
Corneal Neovascularization/*drug therapy
Female
Humans
Male
Middle Aged
Pilot Projects
Prospective Studies
Treatment Outcome
Young Adult
Angiogenesis Inhibitors
Antibodies, Monoclonal, Humanized
Figure
Reference
-
1. Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001; 12:242–249.2. Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000; 41:2514–2522.3. Cursiefen C, Rummelt C, Kuchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000; 19:526–533.4. Chen WL, Lin CT, Lin NT, et al. Subconjunctival injection of bevacizumab (avastin) on corneal neovascularization in different rabbit models of corneal angiogenesis. Invest Ophthalmol Vis Sci. 2009; 50:1659–1665.5. Papathanassiou M, Theodossiadis PG, Liarakos VS, et al. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Ophthalmol. 2008; 145:424–431.6. Yoeruek E, Ziemssen F, Henke-Fahle S, et al. Safety, penetration and efficacy of topically applied bevacizumab: evaluation of eyedrops in corneal neovascularization after chemical burn. Acta Ophthalmol. 2008; 86:322–328.7. Ahmed A, Berati H, Nalan A, Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol. 2009; 37:730–736.8. Carrasco MA. Subconjunctival bevacizumab for corneal neovascularization in herpetic stromal keratitis. Cornea. 2008; 27:743–745.9. Kim SW, Ha BJ, Kim EK, et al. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008; 115:e33–e38.10. Awadein A. Subconjunctival bevacizumab for vascularized rejected corneal grafts. J Cataract Refract Surg. 2007; 33:1991–1993.11. Mansour AM. Treatment of inflamed pterygia or residual pterygial bed. Br J Ophthalmol. 2009; 93:864–865.12. Steinbrook R. The price of sight: ranibizumab, bevacizumab, and the treatment of macular degeneration. N Engl J Med. 2006; 355:1409–1412.13. Rosenfeld PJ. Intravitreal avastin: the low cost alternative to lucentis. Am J Ophthalmol. 2006; 142:141–143.14. Tang XN, Berman AE, Swanson RA, Yenari MA. Digitally quantifying cerebral hemorrhage using Photoshop and Image J. J Neurosci Methods. 2010; 190:240–243.15. Ferrara N, Damico L, Shams N, et al. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006; 26:859–870.16. Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004; 3:391–400.17. Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997; 57:4593–4599.18. Chen Y, Wiesmann C, Fuh G, et al. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999; 293:865–881.19. Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012; 96:1157–1158.20. Klettner A, Roider J. Comparison of bevacizumab, ranibizumab, and pegaptanib in vitro: efficiency and possible additional pathways. Invest Ophthalmol Vis Sci. 2008; 49:4523–4527.21. Carneiro A, Falcao M, Pirraco A, et al. Comparative effects of bevacizumab, ranibizumab and pegaptanib at intravitreal dose range on endothelial cells. Exp Eye Res. 2009; 88:522–527.22. Gharbiya M, Giustolisi R, Allievi F, et al. Choroidal neovascularization in pathologic myopia: intravitreal ranibizumab versus bevacizumab: a randomized controlled trial. Am J Ophthalmol. 2010; 149:458–464.e1.23. Avisar I, Weinberger D, Kremer I. Effect of subconjunctival and intraocular bevacizumab injections on corneal neovascularization in a mouse model. Curr Eye Res. 2010; 35:108–115.24. Mordenti J, Cuthbertson RA, Ferrara N, et al. Comparisons of the intraocular tissue distribution, pharmacokinetics, and safety of 125I-labeled full-length and Fab antibodies in rhesus monkeys following intravitreal administration. Toxicol Pathol. 1999; 27:536–544.25. Gaudreault J, Fei D, Rusit J, et al. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005; 46:726–733.26. Mandalos A, Tsakpinis D, Karayannopoulou G, et al. The effect of subconjunctival ranibizumab on primary pterygium: a pilot study. Cornea. 2010; 29:1373–1379.27. You IC, Kang IS, Lee SH, Yoon KC. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009; 87:653–658.28. Bahar I, Kaiserman I, McAllum P, et al. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res. 2008; 33:23–28.29. Lin CT, Hu FR, Kuo KT, et al. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010; 51:6277–6285.30. Cursiefen C, Hofmann-Rummelt C, Kuchle M, Schlotzer-Schrehardt U. Pericyte recruitment in human corneal angiogenesis: an ultrastructural study with clinicopathological correlation. Br J Ophthalmol. 2003; 87:101–106.31. Galor A, Yoo SH, Piccoli FV, et al. Phase I study of subconjunctival ranibizumab in patients with primary pterygium undergoing pterygium surgery. Am J Ophthalmol. 2010; 149:926–931.e2.32. Kim TI, Chung JL, Hong JP, et al. Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci. 2009; 50:4653–4659.33. Kim EC, Lee WS, Kim MS. The inhibitory effects of bevacizumab eye drops on NGF expression and corneal wound healing in rats. Invest Ophthalmol Vis Sci. 2010; 51:4569–4573.34. Galor A, Yoo SH. Corneal melt while using topical bevacizumab eye drops. Ophthalmic Surg Lasers Imaging. 2010; 03. 09. [Epub]. DOI: 10.3928/15428877-20100215-07.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ranibizumab Injection for Corneal Neovascularization Refractory to Bevacizumab Treatment
- Effect of High-dose Intravitreal Bevacizumab Injection on Refractory Idiopathic Choroidal Neovasculariz
- The Effects of a Subtenoncapsular Injection of Bevacizumab for Ocular Surface Disease With Corneal Neovascularization
- Effect of Bevacizumab and Ranibizumab on the Expression of eNOS in Trabecular Meshwork Cells
- Bi-weekly Subconjunctival Injection of Bevacizumab for Corneal Neovascularization after Burn Injury