Ann Clin Microbiol.
2013 Dec;16(4):168-173. 10.5145/ACM.2013.16.4.168.
Effect of Sodium Citrate on Growth of Bacteria in Blood Culture
- Affiliations
-
- 1Department of Laboratory Medicine, Gyeongsang Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea. sjkim8239@hanmail.net
- 2Department of Emergency Medicine, Gyeongsang Institute of Health Sciences, Gyeongsang National University School of Medicine, Jinju, Korea.
- 3Department of Laboratory Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1501979
- DOI: http://doi.org/10.5145/ACM.2013.16.4.168
Abstract
- BACKGROUND
This study compared the growth of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Streptococcus pneumoniae, and Haemophilus influenzae in blood culture bottles containing anticoagulants, sodium polyanethol sulfonate (SPS) and sodium citrate.
METHODS
One hundred and fifty colony forming units of five different bacterial species were inoculated into standard aerobic (SA) and standard anaerobic (SN) bottles and were combined with 5 mL of human blood in solution with SPS or sodium citrate. Time to detection (TTD) was then monitored using the BacT/Alert 3D system (bioMerieux Inc.).
RESULTS
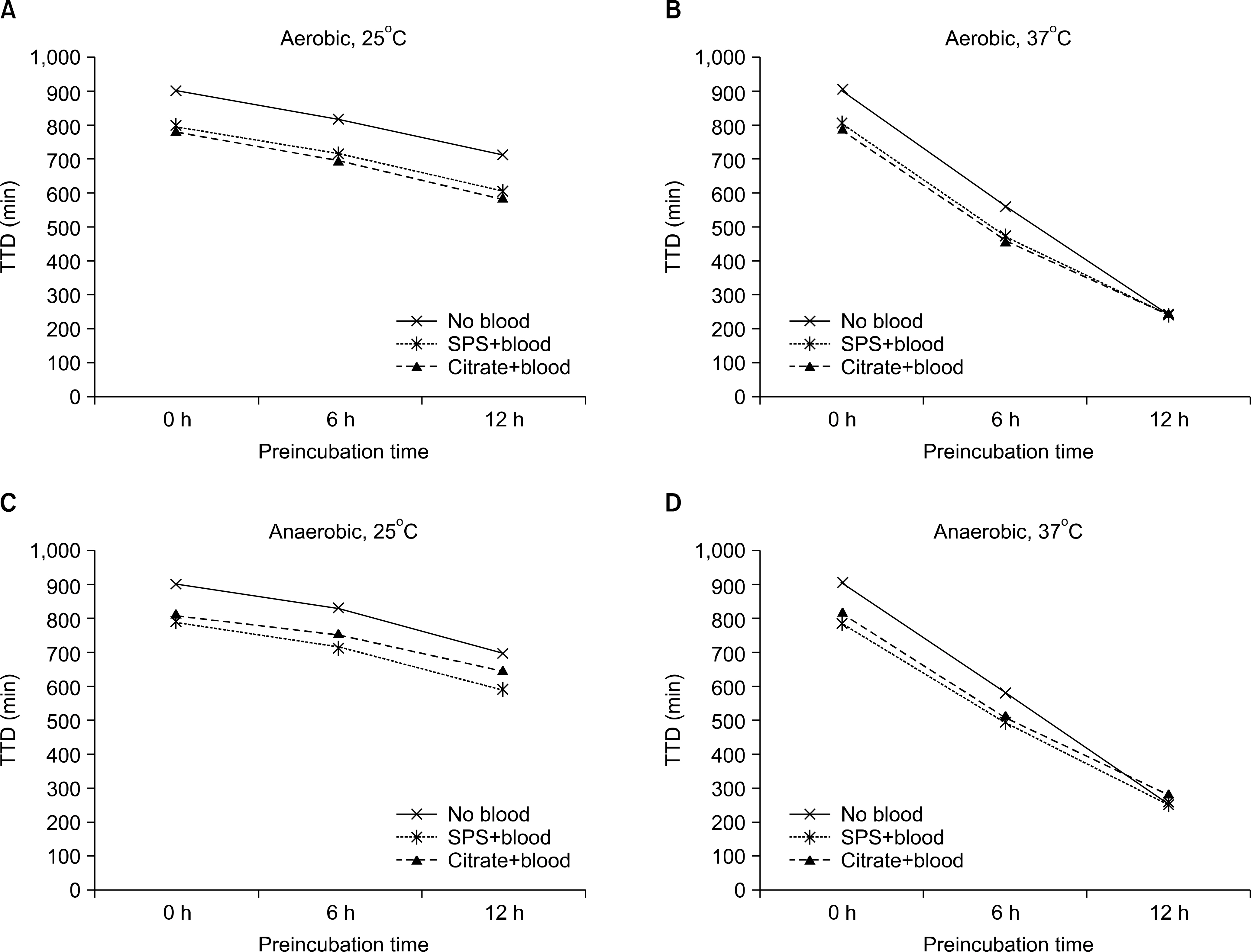
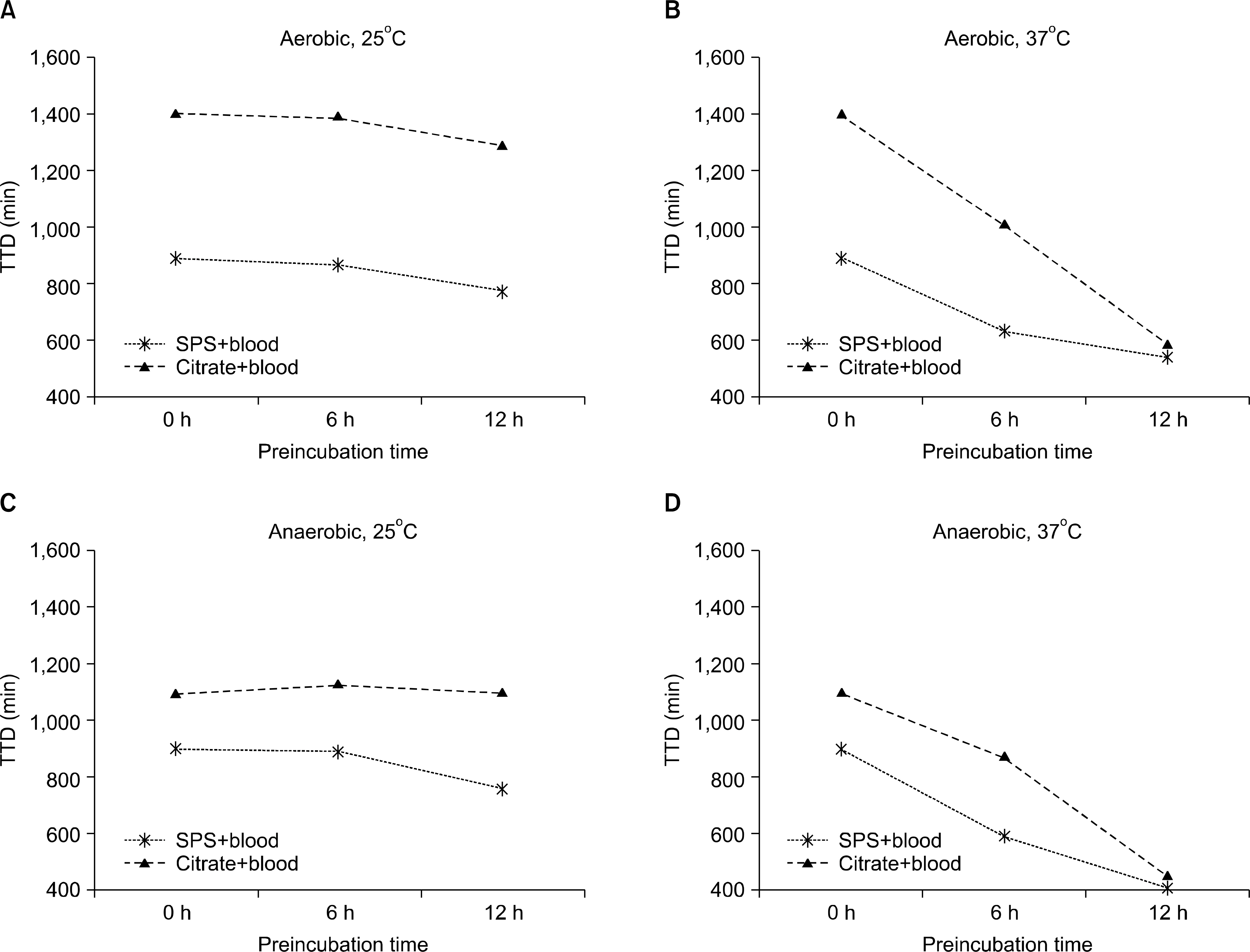
Compared to the bacteria-only controls, cultures containing S. aureus, E. coli, P. aeruginosa, and S. pneumoniae plus SPS blood or citrated blood trended toward reduced TTD in both SA and SN bottles; however, there was no significant difference in TTD between SPS and sodium citrate anticoagulant. Although H. influenzae showed a remarkable difference in TTD between SPS (SA 14.8 h, SN 15.0 h) and sodium citrate (SA 23.5 h, SN 18.3 h), this difference was not statistically significant (P=0.10).
CONCLUSION
Addition of blood enhanced growth of bacteria. All experimental bacteria except H. influenzae showed similar TTD in SPS blood and citrated blood. These results support the usefulness of sodium citrate anticoagulant for artificial inoculation in blood culture bottles.
Keyword
Figure
Reference
-
1.Wilson ML., Mitchell M, et al. Principles and procedures for blood cultures; approved guideline. Wayne, PA; Clinical and Laboratory Standards Institute. 2007.2.Thomson R., Miller J. Specimen Collection, Transport and Pro-Cessing: Bacteriology. Murray PR, Baron EJ, editors. Manual of Clinical Microbiology. Vol. 1:9th ed. Washington, DC: ASM Press;2003. p. 286–330.3.Reimer LG., Wilson ML., Weinstein MP. Update on detection of bacteremia and fungemia. Clin Microbiol Rev. 1997. 10:444–65.
Article4.Kim HJ., Lee NY., Kim S., Shin JH., Kim MN., Kim EC, et al. Characteristics of microorganisms isolated from blood cultures at nine university hospitals in korea during 2009. Korean J Clin Microbiol. 2011. 14:48–54.
Article5.Sautter RL., Bills AR., Lang DL., Ruschell G., Heiter BJ., Bourbeau PP. Effects of delayed-entry conditions on the recovery and detec- tion of microorganisms from BacT/ALERT and BACTEC blood culture bottles. J Clin Microbiol. 2006. 44:1245–9.6.Chapin K., Lauderdale TL. Comparison of Bactec 9240 and Difco ESP blood culture systems for detection of organisms from vials whose entry was delayed. J Clin Microbiol. 1996. 34:543–9.
Article7.Viganò EF., Vasconi E., Agrappi C., Clerici P. Use of simulated blood cultures for time to detection comparison between BacT/ALERT and BACTEC 9240 blood culture systems. Diagn Microbiol Infect Dis. 2002. 44:235–40.
Article8.Akan OA., Yildiz E. Comparison of the effect of delayed entry into 2 different blood culture systems (BACTEC 9240 and BacT/ALERT 3D) on culture positivity. Diagn Microbiol Infect Dis. 2006. 54:193–6.
Article9.Klaerner HG., Eschenbach U., Kamereck K., Lehn N., Wagner H., Miethke T. Failure of an automated blood culture system to detect nonfermentative gram-negative bacteria. J Clin Microbiol. 2000. 38:1036–41.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of thiol broth for the culture of Salmonella typhi and other bacteria from blood
- A Correlation of Prothrombin Time in Citrated Specimens and EDTA Specimens Examined by Owren Method
- Effects of High Concentrations of Sucrose in Blood Culture Media with Special Reference to the Cultivation of Salmonella typhi
- Quantitative Analysis of Weissella cibaria against Periodontopathic Bacteria by Real-time PCR
- A study on the effect of sterilization of the thermometer c three disinfectant sponges