Endocrinol Metab.
2010 Dec;25(4):305-309. 10.3803/EnM.2010.25.4.305.
The Effects of Combined Treatment of Alendronate Plus Active or Plain Vitamin D on the Vitamin D Metabolism and Bone Turnover Markers in Patients with Osteoporosis
- Affiliations
-
- 1Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, Korea. yschung@ajou.ac.kr
- KMID: 1497755
- DOI: http://doi.org/10.3803/EnM.2010.25.4.305
Abstract
- BACKGROUND
The purpose of this study was to evaluate the effects of combined treatment with alendronate plus active or plain vitamin D on the vitamin D metabolism and bone turnover markers in patients with osteoporosis.
METHODS
We investigated 297 osteoporosis outpatients who were treated with Maxmarvil(R) (alendronate 5 mg plus calcitriol 0.5 microg) daily or Fosamax Plus(R) (alendronate 70 mg plus cholecalciferol 2,800 IU) weekly for 1 year. The serum levels of 25(OH)D, parathyroid hormone (PTH), calcium, phosphorus, osteocalcin and N-telopeptide were measured at baseline and after 3, 6, and 12 months of treatment.
RESULTS
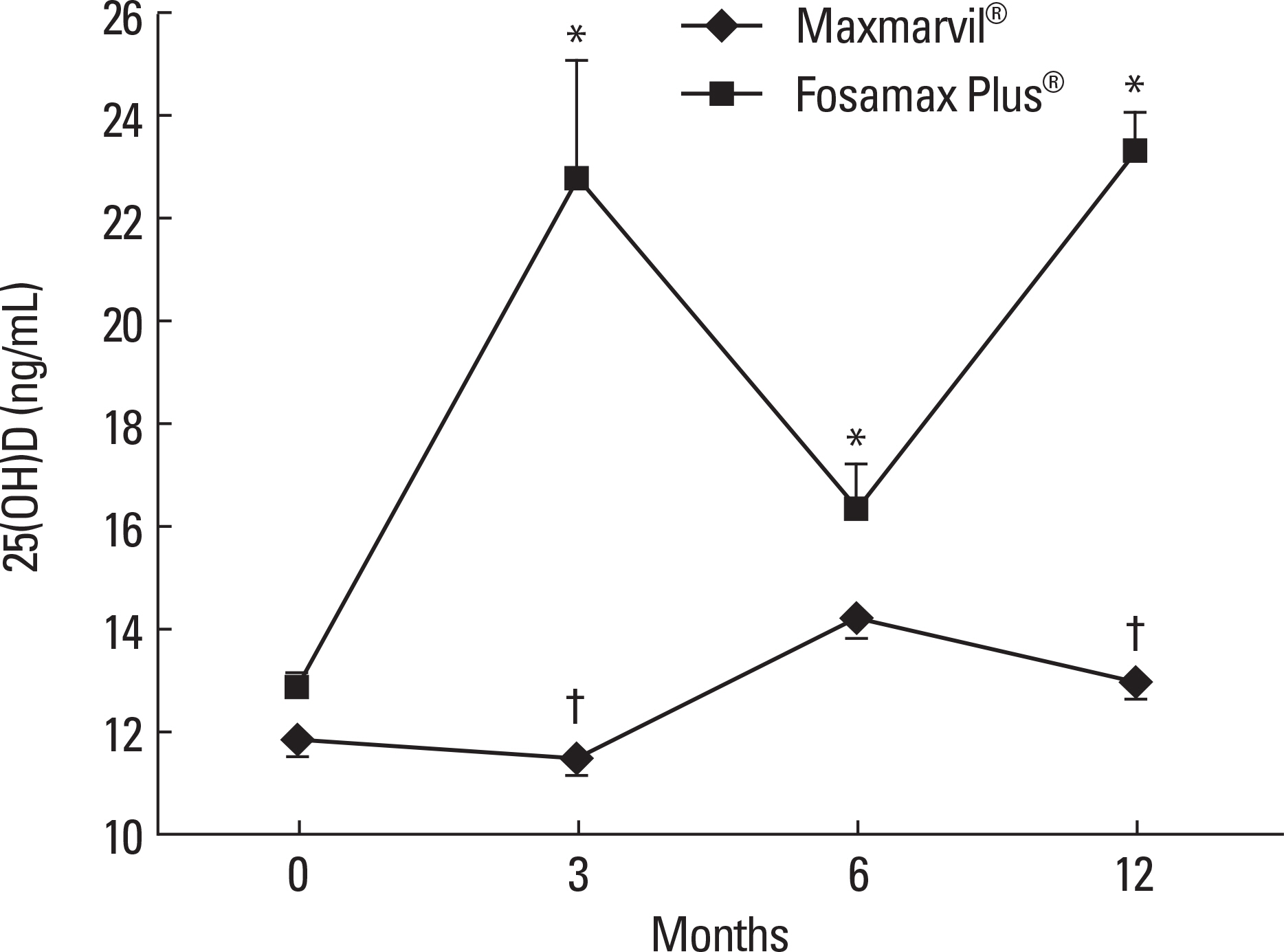
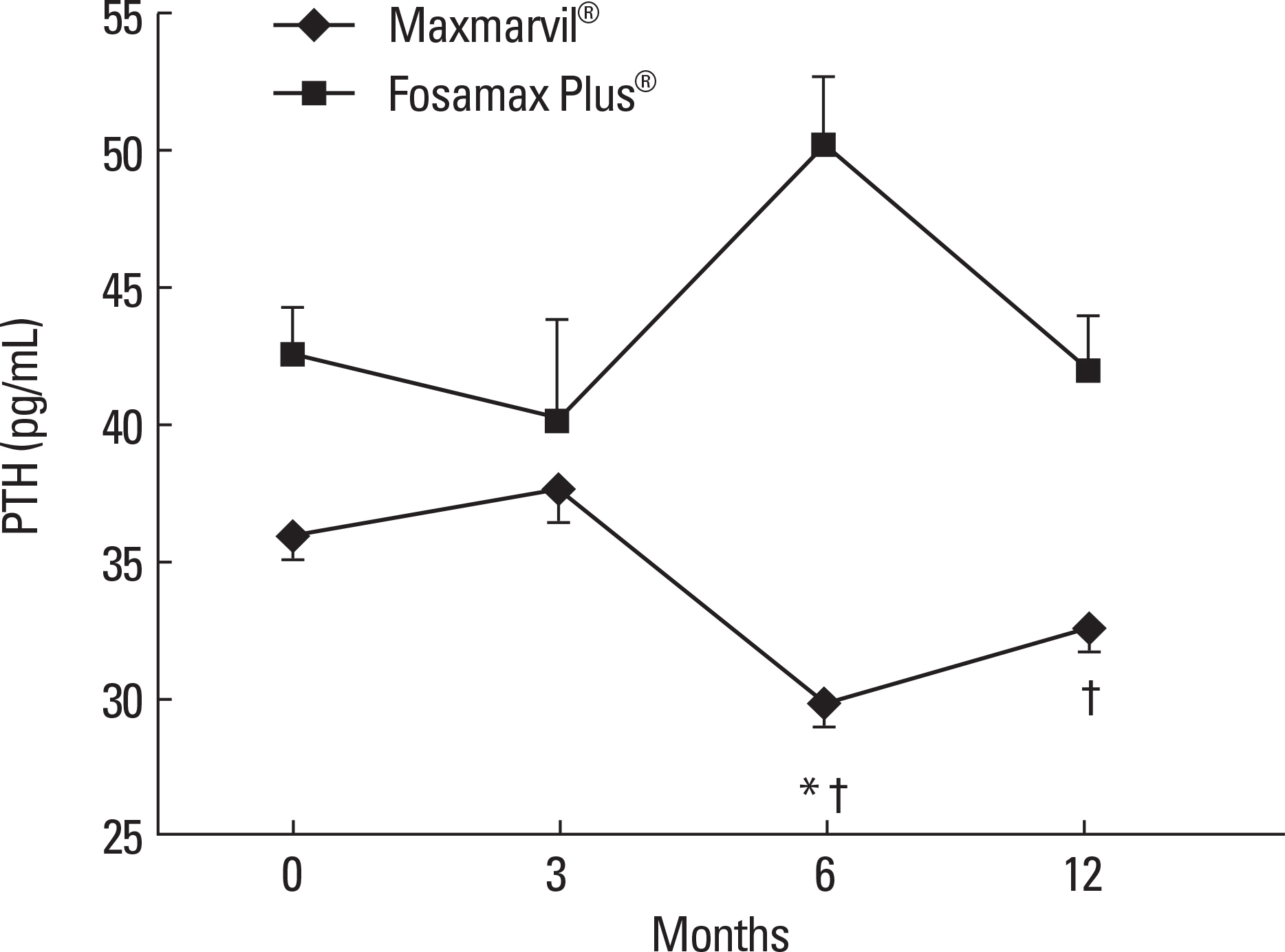
The data of 72 of the 297 patients were analyzed. In the Maxmarvil(R) group (n = 45), the serum PTH significantly decreased by 17% from baseline at 6 months (microd = -6.10; +/- 0.85 SE; P < 0.05) and it remained suppressed to 12 months. The serum 25(OH)D tended to increase, but without significance. In the Fosamax Plus(R) group (n = 27), the serum 25(OH)D significantly increased by 77% from baseline at 3 months (microd = 9.87; +/- 2.32 SE; P < 0.05) and it remained significantly higher than baseline at 6 months (microd = 3.49; +/- 0.86 SE; P < 0.05) and 12 months (microd = 10.47; +/- 0.71 SE; P < 0.001). However, the serum PTH showed no significant decrease. In the Maxmarvil(R) group, the serum osteocalcin significantly decreased by 26% from baseline at 12 months (microd = -5.15; +/- 0.35 SE; P < 0.05), and in the Fosamax Plus(R) group, the serum osteocalcin significantly decreased by 19% from baseline at 6months (microd = -2.64; +/- 0.73 SE; P < 0.05) and it remained suppressed to 12 months (microd = -2.99; +/- 0.37 SE; P = 0.32) without significance.
CONCLUSION
Maxmarvil(R) and Fosamax Plus(R) both improved the bone metabolism in Korean osteoporosis patients. Maxmarvil(R) significantly lowered the serum PTH levels, whereas Fosamax Plus(R) significantly elevated the serum 25(OH)D levels.
Keyword
MeSH Terms
-
Alendronate
Calcitriol
Calcium
Cholecalciferol
Collagen Type I
Diphosphonates
Humans
Osteocalcin
Osteoporosis
Outpatients
Parathyroid Hormone
Peptides
Phosphorus
Vitamin D
Vitamins
Alendronate
Calcitriol
Calcium
Cholecalciferol
Collagen Type I
Diphosphonates
Osteocalcin
Parathyroid Hormone
Peptides
Phosphorus
Vitamin D
Vitamins
Figure
Cited by 1 articles
-
Efficacy and Safety of Weekly Alendronate Plus Vitamin D3 5600 IU versus Weekly Alendronate Alone in Korean Osteoporotic Women: 16-Week Randomized Trial
Kwang Joon Kim, Yong-Ki Min, Jung-Min Koh, Yoon-Sok Chung, Kyoung Min Kim, Dong-Won Byun, In Joo Kim, Mikyung Kim, Sung-Soo Kim, Kyung Wan Min, Ki Ok Han, Hyoung Moo Park, Chan Soo Shin, Sung Hee Choi, Jong Suk Park, Dong Jin Chung, Ji Oh Mok, Hong Sun Baek, Seong-Hwan Moon, Yong Soo Kim, Sung-Kil Lim,
Yonsei Med J. 2014;55(3):715-724. doi: 10.3349/ymj.2014.55.3.715.
Reference
-
References
1. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7–29, 2000: highlights of the conference. South Med J. 94:569–573. 2001.2. Reginster JY. Treatment of postmenopausal osteoporosis. BMJ. 330:859–860. 2005.
Article3. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 87:1080S–1086S. 2008.
Article4. Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 86:1212–1221. 2001.
Article5. National Osteoporosis Foundation. NOF osteoporosis prevention – Vitamin D recommendations. Internet:. http://www.nof.org/prevention/vitaminD.htm. (accessed August 20, 2009).6. Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative metaanalysis. Calcif Tissue Int. 76:176–186. 2005.
Article7. Ringe JD, Schacht E. Prevention and therapy of osteoporosis: the roles of plain Vitamin D and alfacalcidol. Rheumatol Int. 24:189–197. 2004.
Article8. Frediani B, Allegri A, Bisogno S, Marcolongo R. Effects of combined treatment with calcitriol plus alendronate on bone mass and bone turnover in postmenopausal osteoporosis: two years of continuous treatment. Clin Drug Invest. 15:235–244. 1998.9. Khan AA, Bilezikian JP, Kung AW, Ahmed MM, Dubois SJ, Ho AY, Schussheim D, Rubin MR, Shaikh AM, Silverberg SJ, Standish TI, Syed Z, Syed ZA. Alendronate in primary hyperparathyroidism: Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 89:3319–3325. 2004.10. Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 338:736–746. 1998.
Article11. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. Alendronate Phase III Osteoporosis Treatment Study Group. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med. 350:1189–1199. 2004.
Article12. Holick MF. Vitamin D deficiency. N Engl J Med. 357:266–281. 2007.
Article13. Lau KH, Baylink DJ. Vitamin D therapy of osteoporosis: plain vitamin D therapy versus active vitamin D analog (D-hormone) therapy. Calcif Tissue Int. 65:295–306. 1999.
Article14. Recker R, Lips P, Felsenberg D, Lippuner K, Benhamou L, Hawkins F, Delmas PD, Rosen C, Emkey R, Salzmann G, He W, Santora AC. Alendronate with and without cholecalciferol for osteoporosis: Alendronate with and without cholecalciferol for osteoporosis: results of a 15-week randomized controlled trial. Curr Med Res Opin. 22:1745–1755.15. Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, Cowan RA, Downes N. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos Int. 6:284–290. 1996.
Article16. Ringe JD, Farahmand P, Schacht E, Rozehnal A. Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial). Rheumatol Int. 27:425–434. 2007.
Article17. Richy F, Schacht E, Bruyere O, Ethgen O, Gourlay M, Reginster JY. Vitamin D analogs versus native vitamin D in preventing bone loss and osteoporosis-related fractures: a comparative metaanalysis. Calcif Tissue Int. 76:176–186. 2005.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Response: The Effects of Combined Treatment of Alendronate Plus Active or Plain Vitamin D on the Vitamin D Metabolism and Bone Turnover Marker in Patients with Osteoporosis (Endocrinol Metab 25:305-309, 2010, Jee-Hoon Koo et al.)
- Letter: The Effects of Combined Treatment of Alendronate Plus Active or Plain Vitamin D on the Vitamin D Metabolism and Bone Turnover Marker in Patients with Osteoporosis (Endocrinol Metab 25:305-309, 2010, Jee-Hoon Koo et al.)
- Combined Treatment with Vitamin K2 and Bisphosphonate in Postmenopausal Women with Osteoporosis
- Clinical Usefulness of Alendronate for Osteoporosis in Postmenopausal women
- Effects of Vitamin K2 on the Development of Osteopenia in Rats as the Models of Osteoporosis