J Korean Surg Soc.
2009 Dec;77(Suppl):S9-S12. 10.4174/jkss.2009.77.Suppl.S9.
Gastric Glomus Tumor
- Affiliations
-
- 1Department of Surgery, School of Medicine, Pusan National University, Busan, Korea. kdhun@pusan.ac.kr
- 2Department of Internal Medicine, School of Medicine, Pusan National University, Busan, Korea.
- 3Department of Radiology, School of Medicine, Pusan National University, Busan, Korea.
- 4Department of Pathology, School of Medicine, Pusan National University, Busan, Korea.
- KMID: 1464790
- DOI: http://doi.org/10.4174/jkss.2009.77.Suppl.S9
Abstract
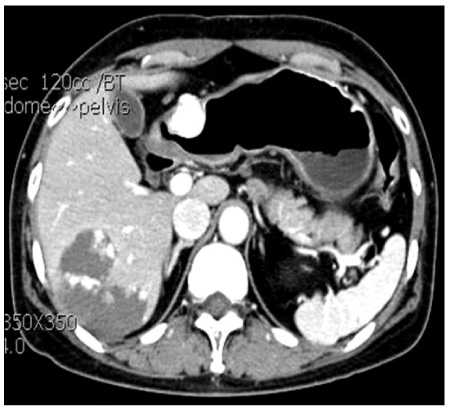
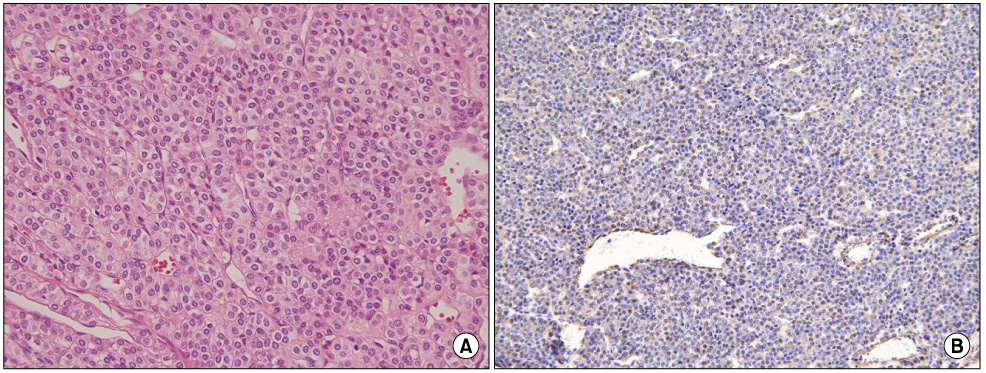
- Gastric glomus tumors are rare submucosal lesions that originate from the modified smooth muscle cells of the glomus body. They usually present as a submucosal tumor on endoscopy and a heterogeneous hypoechoic tumor in the third or fourth sonographic layer of the gastric wall on endoscopic ultrasonography. So they are often confused with other submucosal tumors such as gastrointestinal stromal tumor, schwannoma, and leiomyoma. Immunohistochemistry helps in differentiating glomus tumors from other submucosal tumors. The treatment of choice for these tumors is complete surgical resection. Most of the gastric glomus tumors are essentially benign in nature, so preoperative recognition of this lesion may spare the patient a more extensive resection. Herein, we present three cases of gastric submucosal tumor that were treated by a laparoscopic wedge resection and confirmed as glomus tumor on final pathology.
Keyword
MeSH Terms
Figure
Reference
-
1. Lee HW, Lee JJ, Yang DH, Lee BH. A clinicopathologic study of glomus tumor of the stomach. J Clin Gastroenterol. 2006. 40:717–720.2. Hu XY, Hu CH, Fang XM, Zhang TH. Glomus tumor of the gastric body: helical CT findings. Chin Med J (Engl). 2007. 120:1289–1291.3. Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: a clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol. 2002. 26:301–311.4. Park JP, Park SC, Park CK. A case of gastric glomus tumor. Korean J Gastroenterol. 2008. 52:310–314.5. Lorber J, Kalish J, Farraye FA, Cerda S, Babineau TJ. Glomus tumor of the gastric antrum: case report. Curr Surg. 2005. 62:436–438.6. Ogden WW 2nd, Gottsagen W, Trautman WJ Jr. Glomus tumors of the stomach. Am Surg. 1970. 36:432–436.7. Debol SM, Stanley MW, Mallery S, Sawinski E, Bardales RH. Glomus tumor of the stomach: cytologic diagnosis by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2003. 28:316–321.8. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001. 25:1–12.