J Bacteriol Virol.
2011 Mar;41(1):55-61. 10.4167/jbv.2011.41.1.55.
Recovery and Adsorption Rate of Murine Norovirus Using NanoCeram(R) Filters
- Affiliations
-
- 1Soil & Ground Water Analysis Team, Research & Development Department, Korea Environment Corporation Environmental Research Complex, Incheon, Korea.
- 2Water Supply and Sewerage Research Division, Environmental Infrastructure Research Department, National Institute of Environmental Research Environmental Research Complex, Incheon, Korea. purify@korea.kr
- 3Geum River Environment Research Center, National Institute of Environmental Research, Okcheon, Chungbuk, Korea.
- KMID: 1449863
- DOI: http://doi.org/10.4167/jbv.2011.41.1.55
Abstract
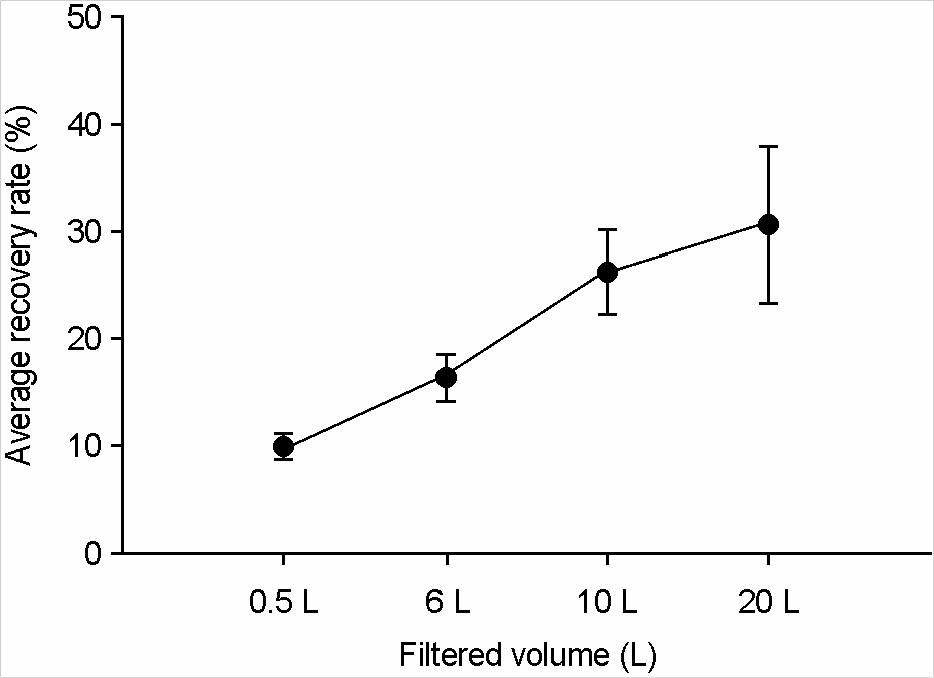
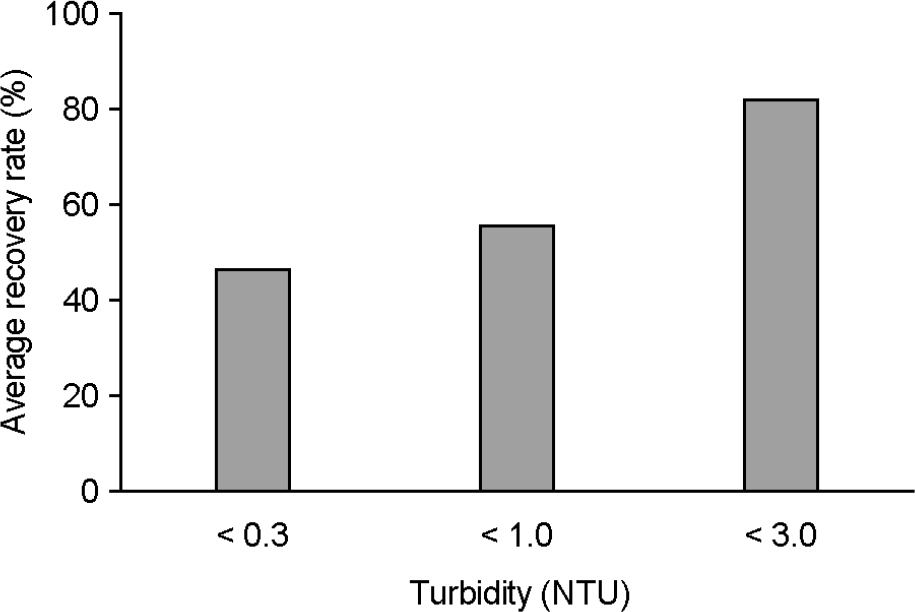
- This study investigated the recovery and absorption rates of murine norovirus, a surrogate for human norovirus, by using NanoCeram(R) filters which served as a tool for recovering viruses. In the study, two types of NanoCeram(R) filters were employed: one was a cartridge type and the other was a disc type (phi 47 mm) whose surface area is 75 times smaller than the cartridge type. The analytical method was the real-time reverse transcription-polymerase chain reaction (RT-PCR). The study found that the average recovery rates of the cartridge type and the disc type were 30.9% and 29.5% respectively. Since these two rates were very close to each other, the adsorption rate of the cartridge type could be predicted with the disc type. Analyzing recovery and absorption rates of the disc type based on different filtered volumes showed that when the volume increased from 0.5 L to 20 L, the average recovery rate rose from 14.78% to 30.41 %, while the average absorption rate dropped from 56.33% to 10.48%. The increase in turbidity from less than 1 NTU to less than 3 NTU raised the average recovery rate from 47.23% to 82.84%.
Keyword
MeSH Terms
Figure
Reference
-
1). Blanton LH., Adams SM., Beard RS., Wei S., Bulens SN., Widdowson MA, et al. Molecular and epidemiologic trends of caliciviruses associated with outbreaks of acute gastroenteritis in the United States, 2000~2004. J Infect Dis. 2006. 193:413–21.
Article2). Hsu CC., Riley LK., Livingston RS. Molecular characterization of three novel murine noroviruses. Virus Genes. 2007. 34:147–55.
Article3). Fankhauser RL., Monroe SS., Noel JS., Humphrey CD., Bresee JS., Parashar UD, et al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002. 186:1–7.
Article4). Bon F., Ambert-Balay K., Giraudon H., Kaplon J., LeGuyader S., Pommepuy M, et al. Molecular epidemiology of caliciviruses detected in sporadic and outbreak cases of gastroenteritis in France from December 1998 to February 2004. J Clin Microbiol. 2005. 43:4659–64.
Article5). Maunula L., Miettinen IT., von Bonsdorff CH. Norovirus outbreaks from drinking water. Emerg Infect Dis. 2005. 11:1716–21.
Article6). Noda M., Fukuda S., Nishio O. Statistical analysis of attack rate in norovirus foodborne outbreaks. Int J Food Microbiol. 2008. 122:216–20.
Article7). Food borne viruses research workshop. 1st ed.Seoul: Korea National Institute of Health;2009.8). Virus survey. Daejeon: Korea Water Research Corporation;2003.9). Lee J., Kim H. Policy directions for resetting the groundwater quality and purification standards. Seoul: Korea Environmental Institute;2007.10). Lee CH., Kim SJ. The genetic diversity of human noroviruses detected in river water in Korea. Water Res. 2008. 42:4477–84.
Article11). Ko G. Molecular characteristics of norovirus and causes of human infection. 3rd ed.News. Seoul: Korean Society for Biochemistry and Molecular Biology;2007. p. 11–5.12). Trujillo AA., McCaustland KA., Zheng DP., Hadley LA., Vaughn G., Adams SM, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol. 2006. 44:1405–12.
Article13). Stals A., Baert L., Botteldoorn N., Werbrouck H., Herman L., Uyttendaele M, et al. Multiplex real-time RT-PCR for simultaneous detection of GI/GII noroviruses and murine norovirus 1. J Virol Methods. 2009. 161:247–53.
Article14). Operating guidance for the organizations to research norovirus in groundwater. Incheon: National Institute of Environmental Research. 2009.15). Bae J., Schwab KJ. Evaluation of murine norovirus, feline calicivirus, poliovirus, and ms2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl Environ Microbiol. 2008. 74:477–84.
Article16). Karim MR., Rhodes ER., Brinkman N., Wymer L., Fout GS. New electropositive filter for concentrating enteroviruses and noroviruses from large volumes of water. Appl Environ Microbiol. 2009. 75:2393–9.
Article17). Lee J., Zoh K., Ko G. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl Environ Microbiol. 2008. 74:2111–7.18). Thurston-Enriquez JA., Haas CN., Jacangelo J., Gerba CP. Inactivation of enteric adenovirus and feline calicivirus by ozone. Water Res. 2005. 39:3650–6.
Article19). Glass RI., Noel J., Ando T., Fankhauser R., Belliot G., Mounts A, et al. The epidemiology of enteric caliciviruses from humans: a reassessment using new diagnostics. J Infect Dis. 2000. 181:S254–61.
Article20). A survey of norovirus contamination in groundwater. Incheon: National Institute of Environmental Research;2008.21). Kim M., Lee H., Chang KO., Ko G. Molecular characterization of murine norovirus isolates from South Korea. Virus Res. 2010. 147:1–6.
Article22). Wobus CE., Thackray LB., Virgin HW 4th. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006. 80:5104–12.
Article23). Cannon JL., Papafragkou E., Park GW., Osborne J., Jaykus LA., Vinje J. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J Food Prot. 2006. 69:2761–5.
Article24). Haramoto E., Katayama H., Utagawa E., Ohgaki S. Recovery of human norovirus from water by virus concentration methods. J Virol Methods. 2009. 160:206–9.
Article25). Sobsey MD., Hickey AR. Effects of humic and fulvic acids on poliovirus concentration from water by microporous filtration. Appl Environ Microbiol. 1985. 49:259–64.
Article26). Sobsey MD., Glass JS. Poliovirus concentration from tap water with electropositive adsorbent filters. Appl Environ Microbiol. 1980. 40:201–10.
Article27). Lee G., Jee Y., Lee C., Lee S. Influence of physicochemical environmental factors on the occurrence of waterborne viruses in Korean surface water. J Bacteriol Virol. 2006. 36:279–85.
Article28). Stetler RE., Ward RL., Waltrip SC. Enteric virus and indicator bacteria levels in a water treatment system modified to reduce trihalomethane production. Appl Environ Microbiol. 1984. 47:319–24.
Article29). Keswick BH., Gerba CP., DuPont HL., Rose JB. Detection of enteric viruses in treated drinking water. Appl Environ Microbiol. 1984. 47:1290–4.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adsorption of desflurane by the silica gel filters in breathing circuits: an in vitro study
- Necrotizing Enterocolitis Associated with Norovirus Infection in a Preterm Infant
- Investigation of Murine Norovirus Replication in RAW264.7 Cells by Strand-specific RT-PCR
- Evaluation of Bedside-use Leukocyte Removal Filter
- Norovirus Associated Cerebellitis in a Previous Healthy 2-year-old Girl