J Korean Surg Soc.
2011 Dec;81(Suppl 1):S59-S63. 10.4174/jkss.2011.81.Suppl1.S59.
Aggressive hilar inflammatory myofibroblastic tumor with hilar bile duct carcinoma in situ
- Affiliations
-
- 1Department of Surgery, Kyung Hee University School of Medicine, Seoul, Korea. sunhyung@chol.com
- 2Department of Pathology, Kyung Hee University School of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Kyung Hee University School of Medicine, Seoul, Korea.
- KMID: 1445788
- DOI: http://doi.org/10.4174/jkss.2011.81.Suppl1.S59
Abstract
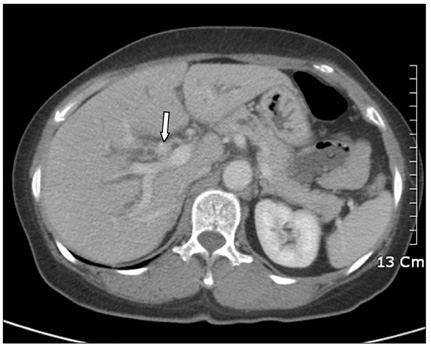
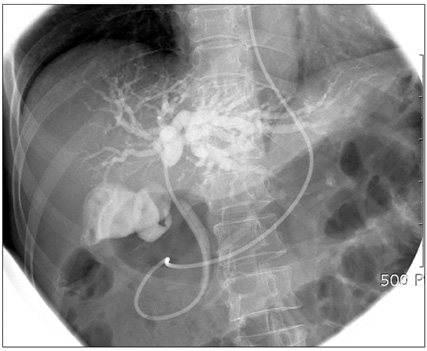
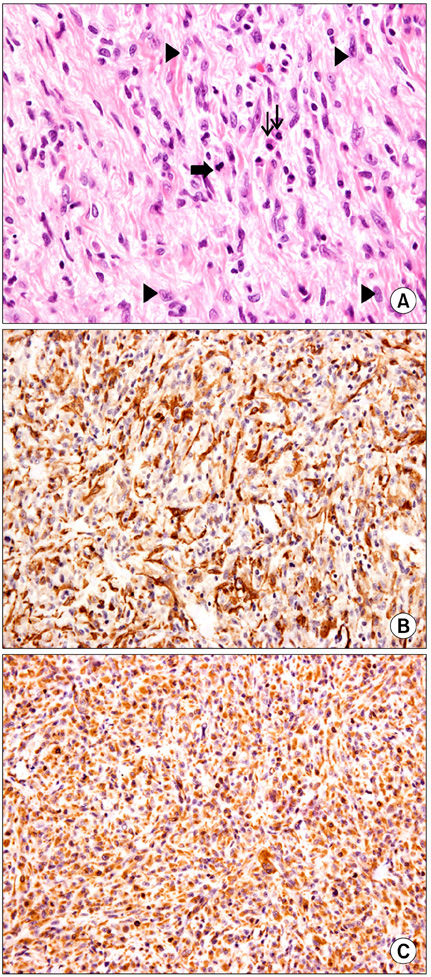
- Inflammatory myofibroblastic tumor (IMT) of the biliary tree is extremely rare and is generally a benign condition, though malignant change is possible. Making a differential diagnosis between this lesion and other malignant conditions is very difficult on preoperative imaging studies. Hence, the final diagnosis of IMT may be made during or after operation depending on the pathologic examination. We treated a 63-year-old woman who received right hepatectomy with caudate lobectomy under the suspicion of hilar cholangiocarcinoma. Frozen biopsy during the operation showed carcinoma in situ and there were stromal cells in the bile duct's resection margins. The postoperative hospital course was uneventful except for minor bile leakage. At postoperative month 4, she developed jaundice, ascites and pleural effusion. Computed tomography images showed a mass-like lesion in the porta hepatis with portal vein thrombosis and a right chest wall mass. Excisional biopsy was done and the pathology report was malignant spindle cell tumor suggestive of an aggressive form of IMT. Her condition rapidly deteriorated regardless of the best supportive care and she expired at postoperative month 5. Further investigation is necessary to clarify the reasons for recurrence and infiltration of this disease.
MeSH Terms
Figure
Reference
-
1. Zamir D, Jarchowsky J, Singer C, Abumoch S, Groisman G, Ammar M, et al. Inflammatory pseudotumor of the liver--a rare entity and a diagnostic challenge. Am J Gastroenterol. 1998. 93:1538–1540.2. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007. 31:509–520.3. Martín Malagón A, López-Tomassetti Fernández E, Arteaga González I, Carrillo Pallarés A, Díaz Luis H. Inflammatory myofibroblastic tumor of the distal bile duct associated with lymphoplasmacytic sclerosing pancreatitis. Case report and review of the literature. Pancreatology. 2006. 6:145–154.4. Rosenbaum L, Fekrazad MH, Rabinowitz I, Vasef MA. Epstein-Barr virus-associated inflammatory pseudotumor of the spleen: report of two cases and review of the literature. J Hematop. 2009. 2:127–131.5. Ashcroft MW, Ng CS, Frost RA, Freeman AH. Biliary inflammatory pseudotumour: report of two cases and review of the literature. Clin Radiol. 2009. 64:449–455.6. Venkataraman S, Semelka RC, Braga L, Danet IM, Woosley JT. Inflammatory myofibroblastic tumor of the hepatobiliary system: report of MR imaging appearance in four patients. Radiology. 2003. 227:758–763.7. Dishop MK, Warner BW, Dehner LP, Kriss VM, Greenwood MF, Geil JD, et al. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003. 25:153–158.8. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.9. Donner LR, Trompler RA, White RR 4th. Progression of inflammatory myofibroblastic tumor (inflammatory pseudotumor) of soft tissue into sarcoma after several recurrences. Hum Pathol. 1996. 27:1095–1098.10. Cook JR, Dehner LP, Collins MH, Ma Z, Morris SW, Coffin CM, et al. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am J Surg Pathol. 2001. 25:1364–1371.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hilar Cholangiocarcinoma: Recent update of radiologic assessment
- Long-term Result of Radical Resection for Hilar Bile Duct Cancer
- Radiological Staging of Hilar Cholangiocarcinoma
- Biliary Tract & Pancreas; A Case of Hilar Cholangiocarcinoma Combined with Carcinoma of the Ampulla of Vater
- Impact of External Beam Radiotherapy after Surgical Resection for Hilar Bile Duct Carcinoma