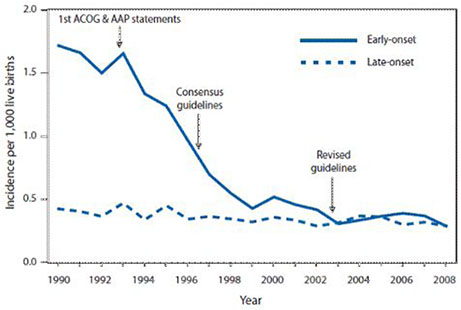
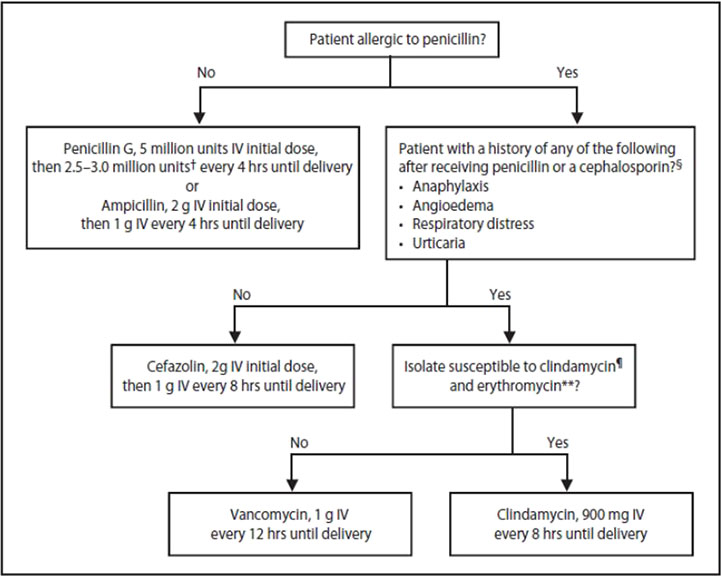
Korean J Pediatr Infect Dis.
2012 Aug;19(2):43-54.
Group B Streptococcal Disease in Korean Neonates
- Affiliations
-
- 1Department of Pediatrics, Kosin University College of Medicine, Busan, Korea. shine707@hanmail.net
Abstract
- Group B streptococcus (GBS) is the leading cause of neonatal sepsis and meningitis in developed countries. This article reviews the neonatal invasive GBS disease, maternal GBS colonization, and prevention strategies in the context of recent epidemiological changes in Korea. Although Korean neonates had been supposed to have low incidence of invasive GBS disease, GBS has been recently reported to be the most common cause of invasive neonatal infection after 1990s. Among Korean pregnant women, GBS carriage rate in the vagina and rectum has been reported to be much lower than that in Western countries. However, it has increased in recent studies. For decision making about preventive strategy for neonatal GBS disease in Korea, further studies are required in terms of the incidence of neonatal GBS infection and serotype distribution. In addition, studies about maternal carriage rate and serotype distribution have to be continued.
MeSH Terms
Figure
Reference
-
1. Edwards MS, Baker CJ. Mandell GL, Bennett JE, Dolin R, editors. Streptococcus agalactiae. Principles and practice of infectious diseases. 2005. 3rd ed. Philadelphia: Elsevier. Churchill Livingstone;2423–2434.2. Edward MS, Nizet V, Baker CJ. Remington JS, Klien JO, Baker CJ, Wilson CB, editors. Group B streptococcal infection. Infectious diseases of the fetus and newborn infant. 2006. 6th ed. Philadelphia: WB Saunders Co;1091–1141.3. Boyer KM, Gadzala CA, Kelly PD, Burd LI, Gotoff SP. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. II. Predictive value of prenatal cultures. J Infect Dis. 1983. 148:802–809.
Article4. Hood M, Janney A, Dameron G. Beta hemolytic streptococcus group B associated with problems of the perinatal period. Am J Obstet Gynecol. 1961. 82:809–818.
Article5. Baker CJ, Barrett FF. Transmission of group B streptococci among parturient women and their neonates. J Pediatr. 1973. 83:919–925.
Article6. Franciosi RA, Knostman JD, Zimmerman RA. Group B streptococcal neonatal and infant infections. J Pediatr. 1973. 82:707–718.
Article7. Kim KA, Shin SM, Choi JH. A nationwide survey on the causative organisms of neonatal sepsis in Korea. J Korean Pediatr Soc. 2002. 45:55–63.8. Lee JH, Cho HK, Kim KH, Kim CH, Kim DS, Kim KN, et al. Etiology of invasive bacterial infections in immunocompetent children in Korea (1996-2005): a retrospective multicenter study. J Korean Med Sci. 2011. 26:174–183.
Article9. Baker CJ, Barrett FF. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974. 230:1158–1160.
Article10. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, et al. Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008. 299:2056–2065.
Article11. Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intra-partum antibiotic prophylaxis. N Engl J Med. 2000. 342:15–20.
Article12. Verani JR, Schrag SJ. Group B streptococcal disease in infants: progress in prevention and continued challenges. Clin Perinatol. 2010. 37:375–392.
Article13. Edwards MS, Baker CJ. Long SS, Pickering LK, Prober CG, editors. Streptococcus agalactiae (Group B Streptococcus). Principles and practice of pediatric infectious diseases revised reprint. 2009. 3rd ed. Philadelphia: Elsevier, Churchill Livingstone;711–716.14. Godambe S, Shah PS, Shah V. Breast milk as a source of late onset neonatal sepsis. Pediatr Infect Dis J. 2005. 24:381–382.
Article15. Hickman ME, Rench MA, Ferrieri P, Baker CJ. Changing epidemiology of group B streptococcal colonization. Pediatrics. 1999. 104:203–209.
Article16. Campbell JR, Hillier SL, Krohn MA, Ferrieri P, Zaleznik DF, Baker CJ. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet Gynecol. 2000. 96:498–503.
Article17. Yancey MK, Schuchat A, Brown LK, Ventura VL, Markenson GR. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet Gynecol. 1996. 88:811–815.
Article18. Kim MW, Jang HO, Chang DY, Cho JR, Kim YA, Choi HM, et al. Group B streptococcal colonization rate in Korean pregnant women. Korean J Obstet Gynecol. 2006. 49:337–344.19. Uh Y, Jang IH, Yoon KJ, Lee CH, Kwon JY, Kim MC. Colonization rates and serotypes of group B streptococci isolated from pregnant women in a Korean tertiary hospital. Eur J Clin Microbiol Infect Dis. 1997. 16:753–756.
Article20. Uh Y, Kwon JY, Jang IH, Yoon KJ, Kim HK. Colonization rate of group B streptococcus in pregnant women and neonates. Korean J Clin Pathol. 1994. 14:447–453.21. Park LS, Seo K, Kim SK, Park YW, Jung HY, Chong YS, et al. A study of group B streptococcal infection in Korean pregnant women. Korean J Obstet Gynecol. 1999. 42:2038–2042.22. Uh Y, Choi SJ, Jang IH, Lee KS, Cho HM, Kwon O, et al. Colonization rate, serotypes, and distributions of macrolide-lincosamide-streptograminB resistant types of group B streptococci in pregnant women. Korean J Clin Microbiol. 2009. 12:174–179.
Article23. Oh CE, Jang HO, Kim NH, Lee J, Choi EH, Lee HJ. Molecular serotyping of group B streptococcus isolated from the pregnant women by polymerase chain reaction and sequence analysis. Korean J Pediatr Infect Dis. 2009. 16:47–53.
Article24. Hong JS, Choi CW, Park KU, Kim SN, Lee HJ, Lee HR, et al. Genital group B streptococcus carrier rate and serotype distribution in Korean pregnant women: implications for group B streptococcal disease in Korean neonates. J Perinat Med. 2010. 38:373–377.
Article25. Yoon HK, Song PJ, Choi KC, Ju JR, Cho BS, Jung SJ. A case of neonatal meningitis by group B streptococcus. J Korean Pediatr Soc. 1984. 27:1011–1017.26. Kim YK, Kwak YH, Kim YJ, Jung HS, Hong JY, Lee HJ. Clinical features of group B β-hemolytic streptococcal infection in infants and children. Korean J Pediatr Infect Dis. 1999. 6:194–202.
Article27. Kim HJ, Lee JW, Lee KY, Lee HS, Hong JH, Hahn SH. Causative organisms in children with bacterial meningitis (1992-2002). J Korean Pediatr Soc. 2003. 46:1085–1088.28. Cho HK, Lee H, Kang JH, Kim KN, Kim DS, Kim YK, et al. The causative organisms of bacterial meningitis in Korean children in 1996-2005. J Korean Med Sci. 2010. 25:895–899.
Article29. Centers for Disease Control and Prevention. Trends in perinatal group B streptococcal disease - United States, 2000-2006. MMWR Morb Mortal Wkly Rep. 2009. 58:109–112.30. Jordan HT, Farley MM, Craig A, Mohle-Boetani J, Harrison LH, Petit S, et al. Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J. 2008. 27:1057–1064.
Article31. Edmond KM, Kortsalioudaki C, Scott S, Schrag SJ, Zaidi AK, Cousens S, et al. Group B streptococcal disease in infants aged younger than 3 months: systematic review and meta-analysis. Lancet. 2012. 379:547–556.
Article32. Kim JS, Jang YT, Kim JD, Park TH, Park JM, Kilgore PE, et al. Incidence of Haemophilus influenzae type b and other invasive diseases in South Korean children. Vaccine. 2004. 22:3952–3962.
Article33. Lee JH, Kim SM, Lee HS, Kim SY, Choi SD, Sung IK, et al. A clinical study of group B streptococcal infection: five year experience. J Korean Soc Neonatol. 2003. 10:226–234.34. Park KH, Kim KH, Kang JH, Kim KN, Kim DS, Kim YK, et al. Current status and clinical presentations of invasive neonatal group B streptococcal infections in Korea. Pediatr Int. 2011. 53:236–239.
Article35. Slotved HC, Kong F, Lambertsen L, Sauer S, Gilbert GL. Serotype IX, a proposed new Streptococcus agalactiae serotype. J Clin Microbiol. 2007. 45:2929–2936.
Article36. Seo YS, Srinivasan U, Oh KY, Shin JH, Chae JD, Kim MY, et al. Changing Molecular Epidemiology of Group B Streptococcus in Korea. J Korean Med Sci. 2010. 25:817–823.
Article37. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986. 314:1665–1669.
Article38. Lim DV, Morales WJ, Walsh AF, Kazanis D. Reduction of morbidity and mortality rates for neonatal group B streptococcal disease through early diagnosis and chemoprophylaxis. J Clin Microbiol. 1986. 23:489–492.
Article39. Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Recomm Rep. 1996. 45(RR-7):1–24.40. Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002. 51(RR-11):1–22.41. Centers for Disease Control and Prevention. Perinatal group B streptococcal disease after universal screening recommendations-United States, 2003-2005. MMWR Morb Mortal Wkly Rep. 2007. 56:701–705.42. Verani JR, McGee L, Schrag SJ. Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC). Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010. 59(RR-10):1–36.43. de Cueto M, Sanchez MJ, Sampedro A, Miranda JA, Herruzo AJ, Rosa-Fraile M. Timing of intrapartum ampicillin and prevention of vertical transmission of group B Streptococcus. Obstet Gynecol. 1998. 91:112–114.
Article44. Lin FY, Brenner RA, Johnson YR, Azimi PH, Philips JB 3rd, Regan JA, et al. The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease. Am J Obstet Gynecol. 2001. 184:1204–1210.
Article45. Fiore Mitchell T, Pearlman MD, Chapman RL, Bhatt-Mehta V, Faix RG. Maternal and transplacental pharmacokinetics of cefazolin. Obstet Gynecol. 2001. 98:1075–1079.
Article46. Kelkar PS, Li JT. Cephalosporin allergy. N Engl J Med. 2001. 345:804–809.
Article47. Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009. 360:2626–2636.
Article48. Melin P. Neonatal group B streptococcal disease: from pathogenesis to preventive strategies. Clin Microbiol Infect. 2011. 17:1294–1303.
Article49. Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976. 294:753–756.
Article50. Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003. 21:3468–3472.
Article51. Baker C, Edwards M. Group B streptococcal conjugate vaccines. Arch Dis Child. 2003. 88:375–378.
Article52. Baker CJ, Paoletti LC, Wessels MR, Guttormsen HK, Rench MA, Hickman ME, et al. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999. 179:142–150.
Article53. Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Carey VJ, Hickman ME, et al. Use of capsular polysaccharide-tetanus toxoid conjugate vaccine for type II group B Streptococcus in healthy women. J Infect Dis. 2000. 182:1129–1138.
Article54. Margarit I, Rinaudo CD, Galeotti CL, Maione D, Ghezzo C, Buttazzoni E, et al. Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J Infect Dis. 2009. 199:108–115.
Article55. Choi KU, Koh SK, Lee JY, Park JH, Hwang SO, Lee BI, et al. Clinical Significance of Group B Streptococcal Infection in Pregnant Women. Korean J Obstet Gynecol. 2002. 45:811–815.56. Lee SH, Park KU, Lee HK, Kim MY, Kim JY, Kwon WK, et al. Perineal colonization rate and antimicrobial susceptibility of group B streptococcus in pregnant and non-pregnant Korean women. Korean J Clin Microbiol. 2009. 12:180–185.
Article57. Seo YS, Srinivasan U, Oh KY, Shin JH, Chae JD, Kim MY, et al. Changing molecular epidemiology of group B streptococcus in Korea. J Korean Med Sci. 2010. 25:817–823.
Article58. Pannaraj PS, Baker CJ. Feigin RD, Cherry J, Demmeler-Harrison GJ, Kaplan SL, editors. Group B streptococcal infections. Feigin and Cherry's textbook of pediatric infectious diseases. 2009. 6th ed. Philadelphia: WB Saunders Co;1239–1257.
Article59. Swingle HM, Bucciarfelli RL, Ayoub EM. Synergy between penicillins and low concentrations of gentamicin in the killing of group B streptococci. J Infect Dis. 1985. 152:515–520.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Group B Streptococcal Infection in Pregnant Women
- Clinical study of group B streptococcal infection in infants less than two months of age
- A Study of Group B Streptococcal Infection in Korean Pregnant Women
- Immunoglobulin and Group B Streptococcal Infection
- Recent development in neonatal Group B streptococcal infection