J Korean Surg Soc.
2012 Dec;83(6):352-359. 10.4174/jkss.2012.83.6.352.
The effectiveness of postoperative neutrophils to lymphocytes ratio in predicting long-term recurrence after stomach cancer surgery
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea. wj2006.choi@samsung.com
- KMID: 1437530
- DOI: http://doi.org/10.4174/jkss.2012.83.6.352
Abstract
- PURPOSE
Immunosuppression is a characteristic of cancer recurrence after curative resection. The neutrophil-to-lymphocyte ratio (NL ratio) in peripheral blood is associated with immune function. However, it is not clear whether the postoperative NL ratio is a predictor for cancer relapse after resection. Thus, we investigated the effectiveness of the short-term postoperative NL ratio in the prediction of disease recurrence within 5 years after stomach cancer surgery by a retrospective chart review.
METHODS
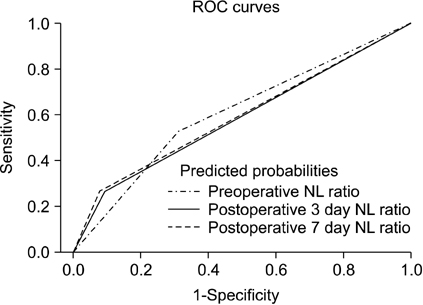
Ninety-three patients with stomach cancer were enrolled. Significant risk factors for cancer recurrence were determined by multivariate Cox regression. Independent variables to increase the NL ratio to >7.7 by postoperative day (POD) 3 were examined by multivariate logistic regression analysis.
RESULTS
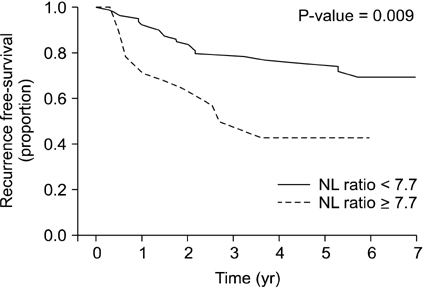
The 5-year risk of cancer recurrence after gastrectomy was 4.2 times higher for patients with a POD3 NL ratio of >7.7 (P = 0.005), 3.4 times higher for normal-weight patients compared with overweight patients (P = 0.008), and 20 times higher for stage III compared with stage 0 according to the tumor-node-metastasis cancer staging system (P = 0.003). The surgical duration (hours) increased the chance of high NL ratio >7.7 (odds ratio, 2.5; P = 0.006).
CONCLUSION
The postoperative NL ratio, especially the POD3 NL ratio, predicts long-term recurrence after stomach cancer surgery.
Keyword
MeSH Terms
Figure
Reference
-
1. Zhu X, Hu XZ, Ma LL, Tian Y. Level of CD4(+) CD25(high) CD127(low/-) regulatory T cells in transitional cell carcinoma patients and its clinical significance. Zhonghua Yi Xue Za Zhi. 2009. 89:2269–2272.2. Standish LJ, Sweet ES, Novack J, Wenner CA, Bridge C, Nelson A, et al. Breast cancer and the immune system. J Soc Integr Oncol. 2008. 6:158–168.3. Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008. 222:101–116.4. Ancuta E, Ancuţa C, Zugun-Eloae F, Iordache C, Chirieac R, Carasevici E. Predictive value of cellular immune response in cervical cancer. Rom J Morphol Embryol. 2009. 50:651–655.5. Yoneyama Y, Ito M, Sugitou M, Kobayashi A, Nishizawa Y, Saito N. Postoperative lymphocyte percentage influences the long-term disease-free survival following a resection for colorectal carcinoma. Jpn J Clin Oncol. 2011. 41:343–347.6. Sarraf KM, Belcher E, Raevsky E, Nicholson AG, Goldstraw P, Lim E. Neutrophil/lymphocyte ratio and its association with survival after complete resection in non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009. 137:425–428.7. Zahorec R. Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001. 102:5–14.8. Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008. 34:55–60.9. Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010. 200:197–203.10. Sharaiha RZ, Halazun KJ, Mirza F, Port JL, Lee PC, Neugut AI, et al. Elevated preoperative neutrophil: lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancer. Ann Surg Oncol. 2011. 18:3362–3369.11. Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. Review article: the role of the perioperative period in recurrence after cancer surgery. Anesth Analg. 2010. 110:1636–1643.12. Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010. 105:106–115.13. Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: immunological basis and implications for psychoneuroimmunology. Brain Behav Immun. 2003. 17:Suppl 1. S27–S36.14. Sugita S, Sasaki A, Iwaki K, Uchida H, Kai S, Shibata K, et al. Prognosis and postoperative lymphocyte count in patients with hepatocellular carcinoma who received intraoperative allogenic blood transfusion: a retrospective study. Eur J Surg Oncol. 2008. 34:339–345.15. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004. 363:157–163.16. Yanai M, Kon A, Kumasaka K, Kawano K. Body mass index variations by age and sex, and prevalence of overweight in Japanese adults. Int J Obes Relat Metab Disord. 1997. 21:484–488.17. Dhar DK, Kubota H, Tachibana M, Kotoh T, Tabara H, Masunaga R, et al. Body mass index determines the success of lymph node dissection and predicts the outcome of gastric carcinoma patients. Oncology. 2000. 59:18–23.18. Gwak MS, Choi SJ, Kim JA, Ko JS, Kim TH, Lee SM, et al. Effects of gender on white blood cell populations and neutrophil-lymphocyte ratio following gastrectomy in patients with stomach cancer. J Korean Med Sci. 2007. 22:Suppl. S104–S108.19. Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2010. 116:5592–5598.20. Atzil S, Arad M, Glasner A, Abiri N, Avraham R, Greenfeld K, et al. Blood transfusion promotes cancer progression: a critical role for aged erythrocytes. Anesthesiology. 2008. 109:989–997.21. Lee SR, Kim HO, Yoo CH. Impact of chronologic age in the elderly with gastric cancer. J Korean Surg Soc. 2012. 82:211–218.22. Lee YY, Choi CH, Sung CO, Do IG, Hub SJ, Kim HJ, et al. Clinical significance of changes in peripheral lymphocyte count after surgery in early cervical cancer. Gynecol Oncol. 2012. 127:107–113.23. Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011. 9:38–43.24. Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, et al. Suppression of cellular immunity by surgical stress. Surgery. 2000. 127:329–336.25. Ferrero E, Ferrero ME, Marni A, Zocchi MR, Stella L, Rugarli C, et al. In vitro effects of halothane on lymphocytes. Eur J Anaesthesiol. 1986. 3:321–330.26. Toft P, Lillevang ST, Tonnesen E, Nielsen CH, Rasmussen JW. The redistribution of granulocytes following E. coli endotoxin induced sepsis. Acta Anaesthesiol Scand. 1994. 38:852–857.27. Tinmouth A, Fergusson D, Yee IC, Hebert PC. ABLE Investigators. Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006. 46:2014–2027.28. Weinberg JA, McGwin G Jr, Griffin RL, Huynh VQ, Cherry SA 3rd, Marques MB, et al. Age of transfused blood: an independent predictor of mortality despite universal leukoreduction. J Trauma. 2008. 65:279–282.29. van de Watering LM, Brand A. Effects of storage of red cells. Transfus Med Hemother. 2008. 35:359–367.30. Nobuoka D, Gotohda N, Kato Y, Takahashi S, Konishi M, Kinoshita T. Influence of excess body weight on the surgical outcomes of total gastrectomy. Surg Today. 2011. 41:928–934.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Postoperative Follow-up of Early Gastric Cancer
- Effects of Gender on White Blood Cell Populations and Neutrophil-Lymphocyte Ratio Following Gastrectomy in Patients with Stomach Cancer
- The Effect of Ginseng on the Nutritional Status and the Immune Functions after Curative Operations on Gastric Carcinoma Patients
- The Changes of Lymphocytes and Subgroups for Postoperative Immunological Response in Gastrointestinal Carcinoma
- The Role of Surgery for the Treatment of Upper Esophageal Cancer