Lab Med Online.
2012 Jan;2(1):1-9. 10.3343/lmo.2012.2.1.1.
Translation: Roadmap for Harmonization of Clinical Laboratory Measurement Procedures
- Affiliations
-
- 1Virginia Commonwealth University, Richmond, VA, USA.
- 2AACC, Washington, USA.
- 3Siemens Healthcare Diagnostics, Newark, DE, USA.
- 4Loyola University Health System, Maywood, IL, USA.
- 5Life Technologies, Benicia, CA, USA.
- 6Ghent University, Ghent, Belgium.
- 7National Institute of Standards and Technology, Gaithersburg, MD, USA.
- 8University of Maryland, Baltimore, MD, USA.
- 9University of Minnesota, Minneapolis, MN, USA.
- 10Medical College of Wisconsin, Milwaukee, WI, USA.
- 11Paul-Ehrlich Institut, Langen, Germany.
- 12Royal Infirmary of Edinburgh, Edinburgh, UK.
- KMID: 1435745
- DOI: http://doi.org/10.3343/lmo.2012.2.1.1
Abstract
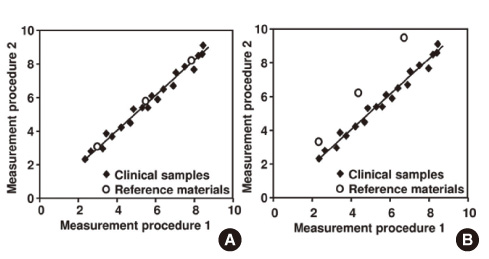
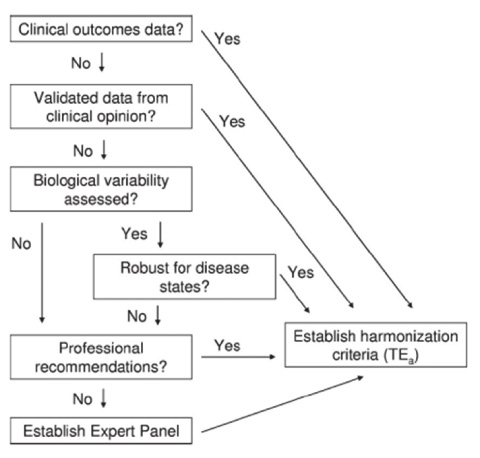
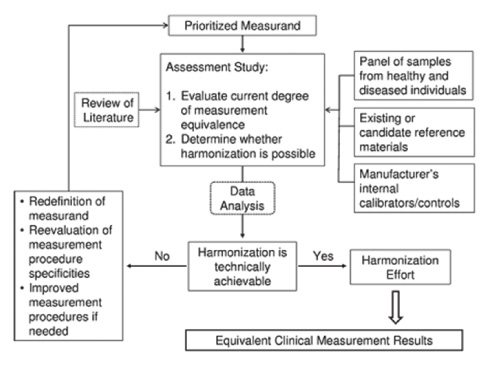
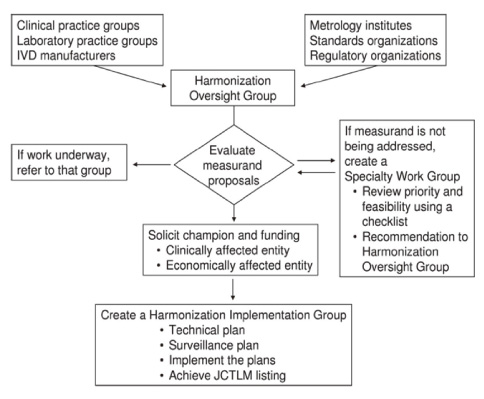
- Results between different clinical laboratory measurement procedures (CLMP) should be equivalent, within clinically meaningful limits, to enable optimal use of clinical guidelines for disease diagnosis and patient management. When laboratory test results are neither standardized nor harmonized, a different numeric result may be obtained for the same clinical sample. Unfortunately, some guidelines are based on test results from a specific laboratory measurement procedure without consideration of the possibility or likelihood of differences between various procedures. When this happens, aggregation of data from different clinical research investigations and development of appropriate clinical practice guidelines will be flawed. A lack of recognition that results are neither standardized nor harmonized may lead to erroneous clinical, financial, regulatory, or technical decisions. Standardization of CLMPs has been accomplished for several measurands for which primary (pure substance) reference materials exist and/or reference measurement procedures (RMPs) have been developed. However, the harmonization of clinical laboratory procedures for measurands that do not have RMPs has been problematic owing to inadequate definition of the measurand, inadequate analytical specificity for the measurand, inadequate attention to the commutability of reference materials, and lack of a systematic approach for harmonization. To address these problems, an infrastructure must be developed to enable a systematic approach for identification and prioritization of measurands to be harmonized on the basis of clinical importance and technical feasibility, and for management of the technical implementation of a harmonization process for a specific measurand.
MeSH Terms
Figure
Reference
-
1. Kohn LT, Corrigan JM, editors. To err is human: building a safer health system. 2000. Washington (DC): National Academy Press;287.2. Vesper HW, Thienpont LM. Traceability in laboratory medicine. Clin Chem. 2009. 55:1067–1075.
Article3. ISO. In vitro diagnostic medical devices-measurement of quantities in biological samples-metrological traceability of values assigned to calibrators and control materials. 2003. 1st ed. Geneva: ISO;ISO 17511:2003(E).4. Armbruster D, Miller RR. The Joint Committee for Traceability in Laboratory Medicine (JCTLM): a global approach to promote the standardisation of clinical laboratory test results. Clin Biochem Rev. 2007. 28:105–113.5. Panteghini M. Traceability as a unique tool to improve standardization in laboratory medicine. Clin Biochem. 2009. 42:236–240.
Article6. Ekins R. Immunoassay standardization. Scand J Clin Lab Invest Suppl. 1991. 205:33–46.
Article7. Thienpont LM. Accuracy in clinical chemistry: who will kiss Sleeping Beauty awake? Clin Chem Lab Med. 2008. 46:1220–1222.8. Zegers I, Keller T, Schreiber W, Sheldon J, Albertini R, Blirup-Jensen S, et al. Characterization of the new serum protein reference material ERM-DA470k/IFCC: value assignment by immunoassay. Clin Chem. 2010. 56:1880–1888.
Article9. Little RR, Rohlfing CL, Tennill AL, Madsen RW, Polonsky KS, Myers GL, et al. Standardization of C-peptide measurements. Clin Chem. 2008. 54:1023–1026.
Article10. Stephan C, Klaas M, Müller C, Schnorr D, Loening SA, Jung K. Interchangeability of measurements of total and free prostate-specific antigen in serum with 5 frequently used assay combinations: an update. Clin Chem. 2006. 52:59–64.
Article11. Miller WG, Thienpont LM, Van Uytfanghe K, Clark PM, Lindstedt P, Nilsson G, et al. Toward standardization of insulin immunoassays. Clin Chem. 2009. 55:1011–1018.
Article12. Thienpont LM, Van Uytfanghe K, Beastall G, Faix JD, Ieiri T, Miller WG, et al. Report of the IFCC Working Group for Standardization of Thyroid Function Tests; part 1: thyroid-stimulating hormone. Clin Chem. 2010. 56:902–911.
Article13. Rose MP. Follicle stimulating hormone international standards and reference preparations for the calibration of immunoassays and bioassays. Clin Chim Acta. 1998. 273:103–117.14. Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, et al. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010. 42:402–408.
Article15. Sturgeon CM, Berger P, Bidart JM, Birken S, Burns C, Norman RJ, et al. Differences in recognition of the 1st WHO international reference reagents for hCG-related isoforms by diagnostic immunoassays for human chorionic gonadotropin. Clin Chem. 2009. 55:1484–1491.
Article16. Thienpont LM, Van Houcke SK. Traceability to a common standard for protein measurements by immunoassay for in-vitro diagnostic purposes. Clin Chim Acta. 2010. 411:2058–2061.
Article17. Schimmel H, Zegers I, Emons H. Standardization of protein biomarker measurements: is it feasible? Scand J Clin Lab Invest Suppl. 2010. 242:27–33.
Article18. Radin N. What is a standard. Clin Chem. 1967. 13:55–76.
Article19. Miller WG, Myers GL, Rej R. Why commutability matters. Clin Chem. 2006. 52:553–554.
Article20. Vesper HW, Miller WG, Myers GL. Reference materials and commutability. Clin Biochem Rev. 2007. 28:139–147.21. Caliendo AM, Shahbazian MD, Schaper C, Ingersoll J, Abdul-Ali D, Boonyaratanakornkit J, et al. A commutable cytomegalovirus calibrator is required to improve the agreement of viral load values between laboratories. Clin Chem. 2009. 55:1701–1710.
Article22. CLSI. CLSI document C53-A. Characterization and qualification of commutable reference materials for laboratory medicine: approved guideline. 2010. Wayne, (PA): CLSI.23. Franzini C, Ceriotti F. Impact of reference materials on accuracy in clinical chemistry. Clin Biochem. 1998. 31:449–457.
Article24. Thienpont LM, Stockl D, Friedecky B, Kratochvila J, Budina M. Trueness verification in European external quality assessment schemes: time to care about the quality of the samples. Scand J Clin Lab Invest. 2003. 63:195–202.
Article25. Miller WG. Specimen materials, target values and commutability for external quality assessment (proficiency testing) schemes. Clin Chim Acta. 2003. 327:25–37.
Article26. Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ. 2003. 326:41–44.
Article27. Roddam AW, Price CP, Allen NE, Ward AM. Assessing the clinical impact of prostate-specific antigen assay variability and nonequimolarity: a simulation study based on the population of the United Kingdom. Clin Chem. 2004. 50:1012–1016.
Article28. NIH consensus statement on management of hepatitis C: 2002. NIH Consens State Sci Statements. 2002. 19:1–46.29. Petersen PH, Sandberg S, Iglesias N, Sölétormos G, Aarsand AK, Brandslund I, et al. 'Likelihood-ratio' and 'odds' applied to monitoring of patients as a supplement to 'reference change value' (RCV). Clin Chem Lab Med. 2008. 46:157–164.
Article30. Karon BS, Boyd JC, Klee GG. Glucose meter performance criteria for tight glycemic control estimated by simulation modeling. Clin Chem. 2010. 56:1091–1097.
Article31. Kenny D, Fraser CG, Hyltoft Petersen P, Kallner A. Strategies to set global analytical quality specifications in laboratory medicine. Consensus agreement. Scand J Clin Lab Invest. 1999. 59:475–585.32. Fraser CG. Biological variation: from principles to practice. 2001. Washington (DC): AACC Press.33. Braga F, Dolci A, Mosca A, Panteghini M. Biological variability of glycated hemoglobin. Clin Chim Acta. 2010. 411:1606–1610.
Article34. Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009. 55:24–38.
Article35. Miller WG, Myers GL, Ashwood ER, Killeen AK, Wang E, Ehlers GW, et al. State of the art in trueness and inter-laboratory harmonization for 10 analytes in general clinical chemistry. Arch Pathol Lab Med. 2008. 132:838–846.
Article36. Palmer-Toy DE. Comparison of pooled fresh frozen serum to proficiency testing material in College of American Pathologists surveys: cortisol and immunoglobulin E. Arch Pathol Lab Med. 2005. 129:305–309.
Article37. Schreiber WE. Comparison of fresh frozen serum to proficiency testing material in College of American Pathologists Surveys: α-fetoprotein, carcinoembryonic antigen, human chorionic gonadotropin, and prostate-specific antigen. Arch Pathol Lab Med. 2005. 129:331–337.
Article38. Blirup-Jensen S. Protein standardization III: method optimization. Basic principles for quantitative determination of human serum proteins on automated instruments based on turbidimetry or nephelometry. Clin Chem Lab Med. 2001. 39:1098–1109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Harmonization: the Sample, the Measurement, and the Report
- Quantitative Evaluation of the Real-World Harmonization Status of Laboratory Test Items Using External Quality Assessment Data
- Validation of the Korean Version of the Patient-Reported Outcomes Information System® Emotional Distress Measures
- Harmonization of laboratory results by data adjustment in multicenter clinical trials
- Monoplane 3D Overlay Roadmap versus Conventional Biplane 2D Roadmap Technique for Neurointervenional Procedures