J Vet Sci.
2012 Jun;13(2):127-137. 10.4142/jvs.2012.13.2.127.
Sequence variations of the bovine prion protein gene (PRNP) in native Korean Hanwoo cattle
- Affiliations
-
- 1Laboratory of Immunology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. hjwoo@snu.ac.kr
- 2Research Institute for Veterinary Science, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea.
- KMID: 1376186
- DOI: http://doi.org/10.4142/jvs.2012.13.2.127
Abstract
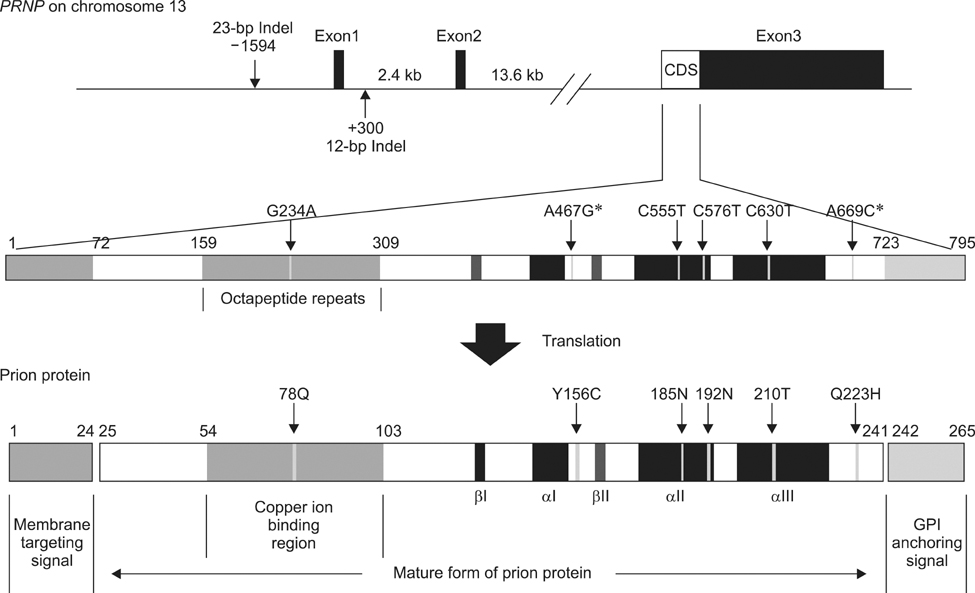
- Bovine spongiform encephalopathy (BSE) is one of the fatal neurodegenerative diseases known as transmissible spongiform encephalopathies (TSEs) caused by infectious prion proteins. Genetic variations correlated with susceptibility or resistance to TSE in humans and sheep have not been reported for bovine strains including those from Holstein, Jersey, and Japanese Black cattle. Here, we investigated bovine prion protein gene (PRNP) variations in Hanwoo cattle [Bos (B.) taurus coreanae], a native breed in Korea. We identified mutations and polymorphisms in the coding region of PRNP, determined their frequency, and evaluated their significance. We identified four synonymous polymorphisms and two non-synonymous mutations in PRNP, but found no novel polymorphisms. The sequence and number of octapeptide repeats were completely conserved, and the haplotype frequency of the coding region was similar to that of other B. taurus strains. When we examined the 23-bp and 12-bp insertion/deletion (indel) polymorphisms in the non-coding region of PRNP, Hanwoo cattle had a lower deletion allele and 23-bp del/12-bp del haplotype frequency than healthy and BSE-affected animals of other strains. Thus, Hanwoo are seemingly less susceptible to BSE than other strains due to the 23-bp and 12-bp indel polymorphisms.
MeSH Terms
Figure
Reference
-
1. Baylis M, Goldmann W. The genetics of scrapie in sheep and goats. Curr Mol Med. 2004. 4:385–396.
Article2. Belay ED. Transmissible spongiform encephalopathies in humans. Annu Rev Microbiol. 1999. 53:283–314.
Article3. Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, Cousens S, Fraser H, Bostock CJ. Transmissions to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature. 1997. 389:498–501.
Article4. Brunelle BW, Greenlee JJ, Seabury CM, Brown CE 2nd, Nicholson EM. Frequencies of polymorphisms associated with BSE resistance differ significantly between Bos taurus, Bos indicus, and composite cattle. BMC Vet Res. 2008. 4:36.5. Brunelle BW, Hamir AN, Baron T, Biacabe AG, Richt JA, Kunkle RA, Cutlip RC, Miller JM, Nicholson EM. Polymorphisms of the prion gene promoter region that influence classical bovine spongiform encephalopathy susceptibility are not applicable to other transmissible spongiform encephalopathies in cattle. J Anim Sci. 2007. 85:3142–3147.
Article6. Castilla J, Gutiérrez-Adán A, Brun A, Pintado B, Salguero FJ, Parra B, Segundo FD, Ramírez MA, Rábano A, Cano MJ, Torres JM. Transgenic mice expressing bovine PrP with a four extra repeat octapeptide insert mutation show a spontaneous, non-transmissible, neurodegenerative disease and an expedited course of BSE infection. FEBS Lett. 2005. 579:6237–6246.
Article7. Choi CJ, Kanthasamy A, Anantharam V, Kanthasamy AG. Interaction of metals with prion protein: possible role of divalent cations in the pathogenesis of prion diseases. Neurotoxicology. 2006. 27:777–787.
Article8. Goldmann W, Hunter N, Smith G, Foster J, Hope J. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J Gen Virol. 1994. 75:989–995.
Article9. Haase B, Doherr MG, Seuberlich T, Drögemüller C, Dolf G, Nicken P, Schiebel K, Ziegler U, Groschup MH, Zurbriggen A, Leeb T. PRNP promoter polymorphisms are associated with BSE susceptibility in Swiss and German cattle. BMC Genet. 2007. 8:15.10. Heaton MP, Keele JW, Harhay GP, Richt JA, Koohmaraie M, Wheeler TL, Shackelford SD, Casas E, King DA, Sonstegard TS, Van Tassell CP, Neibergs HL, Chase CC Jr, Kalbfleisch TS, Smith TP, Clawson ML, Laegreid WW. Prevalence of the prion protein gene E211K variant in U.S. cattle. BMC Vet Res. 2008. 4:25.
Article11. Hresko S, Mojzis M, Tkacikova L. Prion protein gene polymorphism in healthy and BSE-affected Slovak cattle. J Appl Genet. 2009. 50:371–374.
Article12. Hur SJ, Park GB, Joo ST. A comparison of the meat qualities from the Hanwoo (Korean native cattle) and Holstein steer. Food Bioprocess Tech. 2008. 1:196–200.
Article13. Inoue S, Tanaka M, Horiuchi M, Ishiguro N, Shinagawa M. Characterization of the bovine prion protein gene: the expression requires interaction between the promoter and intron. J Vet Med Sci. 1997. 59:175–183.
Article14. Jeong BH, Lee YJ, Kim NH, Carp RI, Kim YS. Genotype distribution of the prion protein gene (PRNP) promoter polymorphisms in Korean cattle. Genome. 2006. 49:1539–1544.
Article15. Jeong BH, Sohn HJ, Lee JO, Kim NH, Kim JI, Lee SY, Cho IS, Joo YS, Carp RI, Kim YS. Polymorphisms of the prion protein gene (PRNP) in Hanwoo (Bos Taurus coreanae) and Holstein cattle. Genes Genet Syst. 2005. 80:303–308.
Article16. Juling K, Schwarzenbacher H, Williams JL, Fries R. A major genetic component of BSE susceptibility. BMC Biol. 2006. 4:33.
Article17. Jung HY, Park JS, Park YJ, Kim YJ, Kimm K, Koh IS. HapAnalyzer: minimum haplotype analysis system for association studies. Genomics Inform. 2004. 2:107–109.18. Kim Y, Kim JB, Sohn H, Lee C. A national survey on the allelic, genotypic, and haplotypic distribution of PRNP insertion and deletion polymorphisms in Korean cattle. J Genet. 2009. 88:99–103.
Article19. Krasemann S, Zerr I, Weber T, Poser S, Kretzschmar H, Hunsmann G, Bodemer W. Prion disease associated with a novel nine octapeptide repeat insertion in the PRNP gene. Brain Res Mol Brain Res. 1995. 34:173–176.
Article20. Lee DK, Suh D, Edenberg HJ, Hur MW. POZ domain transcription factor, FBI-1, represses transcription of ADH5/FDH by interacting with the zinc finger and interfering with DNA binding activity of Sp1. J Biol Chem. 2002. 277:26761–26768.
Article21. McKenzie D, Bartz J, Mirwald J, Olander D, Marsh R, Aiken J. Reversibility of scrapie inactivation is enhanced by copper. J Biol Chem. 1998. 273:25545–25547.
Article22. Mead S. Prion disease genetics. Eur J Hum Genet. 2006. 14:273–281.
Article23. Msalya G, Shimogiri T, Nishitani K, Okamoto S, Kawabe K, Minesawa M, Maeda Y. Indels within promoter and intron 1 of bovine prion protein gene modulate the gene expression levels in the medulla oblongata of two Japanese cattle breeds. Anim Genet. 2010. 41:218–221.
Article24. Nakamitsu S, Miyazawa T, Horiuchi M, Onoe S, Ohoba Y, Kitagawa H, Ishiguro N. Sequence variation of bovine prion protein gene in Japanese cattle (Holstein and Japanese Black). J Vet Med Sci. 2006. 68:27–33.
Article25. Nicholson EM, Brunelle BW, Richt JA, Kehrli ME Jr, Greenlee JJ. Identification of a heritable polymorphism in bovine PRNP associated with genetic transmissible spongiform encephalopathy: evidence of heritable BSE. PLoS One. 2008. 3:e2912.26. Novakofski J, Brewer MS, Mateus-Pinilla N, Killefer J, McCusker RH. Prion biology relevant to bovine spongiform encephalopathy. J Anim Sci. 2005. 83:1455–1476.27. Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982. 216:136–144.
Article28. Prusiner SB, editor. Prion Biology and Diseases. 2004. 2nd ed. NewYork: Cold Spring Harbor Laboratory Press;1–87.29. Prusiner SB. Prions. Proc Natl Acad Sci USA. 1998. 95:13363–13383.
Article30. Qin LH, Zhao YM, Bao YH, Bai WL, Chong J, Zhang GL, Zhang JB, Zhao ZH. Polymorphism of the prion protein gene (PRNP) in two Chinese indigenous cattle breeds. Mol Biol Rep. 2011. 38:4197–4204.
Article31. Ryan AM, Womack JE. Somatic cell mapping of the bovine prion protein gene and restriction fragment length polymorphism studies in cattle and sheep. Anim Genet. 1993. 24:23–26.
Article32. Sander P, Hamann H, Drögemüller C, Kashkevich K, Schiebel K, Leeb T. Bovine prion protein gene (PRNP) promoter polymorphisms modulate PRNP expression and may be responsible for differences in bovine spongiform encephalopathy susceptibility. J Biol Chem. 2005. 280:37408–37414.
Article33. Sander P, Hamann H, Pfeiffer I, Wemheuer W, Brenig B, Groschup MH, Ziegler U, Distl O, Leeb T. Analysis of sequence variability of the bovine prion protein gene (PRNP) in German cattle breeds. Neurogenetics. 2004. 5:19–25.
Article34. Schläpfer J, Saitbekova N, Gaillard C, Dolf G. A new allelic variant in the bovine prion protein gene (PRNP) coding region. Anim Genet. 1999. 30:386–387.
Article35. Scott MR, Will R, Ironside J, Nguyen HO, Tremblay P, DeArmond SJ, Prusiner SB. Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci USA. 1999. 96:15137–15142.
Article36. Seabury CM, Womack JE, Piedrahita J, Derr JN. Comparative PRNP genotyping of U.S. cattle sires for potential association with BSE. Mamm Genome. 2004. 15:828–833.
Article37. Viles JH, Cohen FE, Prusiner SB, Goodin DB, Wright PE, Dyson HJ. Copper binding to the prion protein: structural implications of four identical cooperative binding sites. Proc Natl Acad Sci USA. 1999. 96:2042–2047.
Article38. Wadsworth JDF, Asante EA, Desbruslais M, Linehan JM, Joiner S, Gowland I, Welch J, Stone L, Lloyd SE, Hill AF, Brandner S, Collinge J. Human prion protein with valine 129 prevents expression of variant CJD phenotype. Science. 2004. 306:1793–1796.
Article39. Zhao H, Wang XY, Zou W, Zhang YP. Prion protein gene (PRNP) polymorphisms in native Chinese cattle. Genome. 2010. 53:138–145.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biological characteristics of Chinese hamster ovary cells transfected with bovine Prnp
- Genetic Studies in Human Prion Diseases
- Identification of single-nucleotide polymorphisms of the prion protein gene in sika deer (Cervus nippon laiouanus)
- Identification of Genomic Differences between Hanwoo and Holstein Breeds Using the Illumina Bovine SNP50 BeadChip
- Molecular analysis of prion protein gene (PRNP) in Korean patients with Creutzfeldt-Jakob disease