Korean J Gastroenterol.
2012 Mar;59(3):232-238. 10.4166/kjg.2012.59.3.232.
Could Nafamostat or Gabexate Prevent the Post Endoscopic Retrograde Cholangiopancreatography Pancreatitis?
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University School of Medicine, Daegu, Korea. minky1973@Kornet.net
- KMID: 1245208
- DOI: http://doi.org/10.4166/kjg.2012.59.3.232
Abstract
- BACKGROUND/AIMS
ERCP is the most common procedure for the diagnosis and treatment of bile duct and pancreatic disease, but Post-ERCP pancreatitis makes poor outcome in some cases. The protease inhibitors, nafamostat and gabexate, have been used to prevent pancreatitis related to ERCP, but there is some debate. We tried to evaluate the efficacy of gabexate and nafamostat for the prevention of post-ERCP pancreatitis.
METHODS
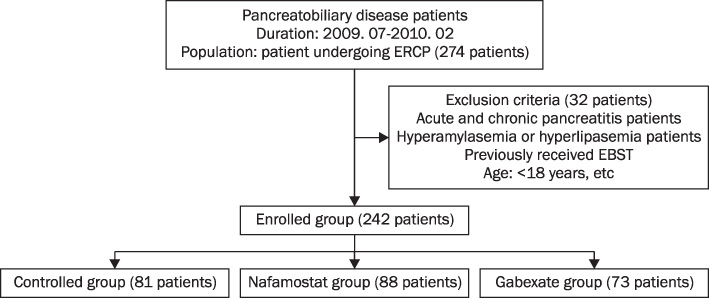
Two hundred forty two patients (73 patients in the gabexate group, 88 patients in the nafamostat group and 81 patients in the placebo group) were included in the study after selective exclusion. The incidence of pancreatitis after ERCP was compared among groups.
RESULTS
The incidence of pancreatitis were 6.8% in the gabexate group, 5.7% in the nafamostat group and 6.2% in the placebo group (p=0.954).
CONCLUSIONS
There was no meaningful difference among the gabexate, nafamostat and placebo group.
MeSH Terms
Figure
Reference
-
1. Manes G, Ardizzone S, Lombardi G, Uomo G, Pieramico O, Porro GB. Efficacy of postprocedure administration of gabexate mesylate in the prevention of post-ERCP pancreatitis: a randomized, controlled, multicenter study. Gastrointest Endosc. 2007. 65:982–987.2. Cheon YK, Cho KB, Watkins JL, et al. Efficacy of diclofenac in the prevention of post-ERCP pancreatitis in predominantly high-risk patients: a randomized double-blind prospective trial. Gastrointest Endosc. 2007. 66:1126–1132.3. Zheng M, Chen Y, Yang X, Li J, Zhang Y, Zeng Q. Gabexate in the prophylaxis of post-ERCP pancreatitis: a meta-analysis of randomized controlled trials. BMC Gastroenterol. 2007. 7:6.4. Murray B, Carter R, Imrie C, Evans S, O'Suilleabhain C. Diclofenac reduces the incidence of acute pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2003. 124:1786–1791.5. Sotoudehmanesh R, Khatibian M, Kolahdoozan S, Ainechi S, Malboosbaf R, Nouraie M. Indomethacin may reduce the incidence and severity of acute pancreatitis after ERCP. Am J Gastroenterol. 2007. 102:978–983.6. Andriulli A, Caruso N, Quitadamo M, et al. Antisecretory vs. antiproteasic drugs in the prevention of post-ERCP pancreatitis: the evidence-based medicine derived from a meta-analysis study. JOP. 2003. 4:41–48.7. Andriulli A, Leandro G, Federici T, et al. Prophylactic administration of somatostatin or gabexate does not prevent pancreatitis after ERCP: an updated meta-analysis. Gastrointest Endosc. 2007. 65:624–632.8. Pezzilli R, Romboli E, Campana D, Corinaldesi R. Mechanisms involved in the onset of post-ERCP pancreatitis. JOP. 2002. 3:162–168.9. Freeman ML, Guda NM. Prevention of post-ERCP pancreatitis: a comprehensive review. Gastrointest Endosc. 2004. 59:845–864.10. Cavallini G, Tittobello A, Frulloni L, Masci E, Mariana A, Di Francesco V. Gabexate in digestive endoscopy--Italian Group. Gabexate for the prevention of pancreatic damage related to endoscopic retrograde cholangiopancreatography. N Engl J Med. 1996. 335:919–923.11. Jeen YT, Chun HJ, Lee JW, et al. Effects of gabexate mesilate for the prevention of pancreatic damage related to ERCP. Korean J Gastrointest Endosc. 2000. 21:534–542.12. Fujii S, Hitomi Y. New synthetic inhibitors of C1r, C1 esterase, thrombin, plasmin, kallikrein and trypsin. Biochim Biophys Acta. 1981. 661:342–345.13. Iwaki M, Ino Y, Motoyoshi A, et al. Pharmacological studies of FUT-175, nafamostat mesilate. V. Effects on the pancreatic enzymes and experimental acute pancreatitis in rats. Jpn J Pharmacol. 1986. 41:155–162.14. Singh VP, Chari ST. Protease inhibitors in acute pancreatitis: lessons from the bench and failed clinical trials. Gastroenterology. 2005. 128:2172–2174.15. Seta T, Noguchi Y, Shimada T, Shikata S, Fukui T. Treatment of acute pancreatitis with protease inhibitors: a meta-analysis. Eur J Gastroenterol Hepatol. 2004. 16:1287–1293.16. Andriulli A, Leandro G, Clemente R, et al. Meta-analysis of somatostatin, octreotide and gabexate mesilate in the therapy of acute pancreatitis. Aliment Pharmacol Ther. 1998. 12:237–245.17. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991. 37:383–393.18. McCune WS, Shorb PE, Moscovitz H. Endoscopic cannulation of the ampulla of vater: a preliminary report. Ann Surg. 1968. 167:752–756.19. Skude G, Wehlin L, Maruyama T, Ariyama J. Hyperamylasaemia after duodenoscopy and retrograde cholangiopancreatography. Gut. 1976. 17:127–132.20. Chen YK, Foliente RL, Santoro MJ, Walter MH, Collen MJ. Endoscopic sphincterotomy-induced pancreatitis: increased risk associated with nondilated bile ducts and sphincter of Oddi dysfunction. Am J Gastroenterol. 1994. 89:327–333.21. Gottlieb K, Sherman S. ERCP and biliary endoscopic sphincterotomy-induced pancreatitis. Gastrointest Endosc Clin N Am. 1998. 8:87–114.22. Freeman ML. Post-ERCP pancreatitis: patient and technique-related risk factors. JOP. 2002. 3:169–176.23. Testoni PA. Preventing post-ERCP pancreatitis: where are we? JOP. 2003. 4:22–32.24. Andriulli A, Clemente R, Solmi L, et al. Gabexate or somatostatin administration before ERCP in patients at high risk for post-ERCP pancreatitis: a multicenter, placebo-controlled, randomized clinical trial. Gastrointest Endosc. 2002. 56:488–495.25. Andriulli A, Solmi L, Loperfido S, et al. Prophylaxis of ERCP-related pancreatitis: a randomized, controlled trial of somatostatin and gabexate mesylate. Clin Gastroenterol Hepatol. 2004. 2:713–718.26. Keck T, Balcom JH, Antoniu BA, et al. Regional effects of nafamostat, a novel potent protease and complement inhibitor, on severe necrotizing pancreatitis. Surgery. 2001. 130:175–181.27. Chang JH, Lee IS, Kim HK, et al. Nafamostat for prophylaxis against post-endoscopic retrograde cholangiopancreatography pancreatitis compared with gabexate. Gut Liver. 2009. 3:205–210.28. Cortesi R, Ascenzi P, Colasanti M, et al. Cross-enzyme inhibition by gabexate mesylate: formulation and reactivity study. J Pharm Sci. 1998. 87:1335–1340.29. Pitkäranta P, Kivisaari L, Nordling S, Nuutinen P, Schroder T. Vascular changes of pancreatic ducts and vessels in acute necrotizing, and in chronic pancreatitis in humans. Int J Pancreatol. 1991. 8:13–22.30. Nuutinen P, Kivisaari L, Schröder T. Contrast-enhanced computed tomography and microangiography of the pancreas in acute human hemorrhagic/necrotizing pancreatitis. Pancreas. 1988. 3:53–60.31. Freeman ML, Nelson DB, Sherman S, et al. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996. 335:909–918.32. Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest Endosc. 2001. 54:425–434.33. Loperfido S, Angelini G, Benedetti G, et al. Major early complications from diagnostic and therapeutic ERCP: a prospective multicenter study. Gastrointest Endosc. 1998. 48:1–10.34. Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002. 56:652–656.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nafamostat for Prophylaxis against Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis Compared with Gabexate
- Risk Factors for Post-ERCP Pancreatitis after Needle Knife Sphincterotomy following Repeated Probing
- Prevention of Post-endoscopic Retrograde Cholangiopancreatography Pancreatitis: An Endoscopic Perspective
- The Use of Gabexate Mesylate and Ulinastatin for the Prevention of Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis
- Gabexate for the Prevention of Pancreatic Complications Following Endoscopic Retrograde Cholangiopancreatography