Korean J Radiol.
2011 Jun;12(3):269-279. 10.3348/kjr.2011.12.3.269.
Liver Flukes: the Malady Neglected
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul 135-710, Korea. jhlim@smc.samsung.co.kr
- KMID: 1122324
- DOI: http://doi.org/10.3348/kjr.2011.12.3.269
Abstract
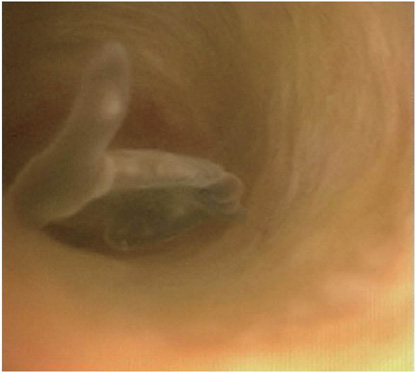
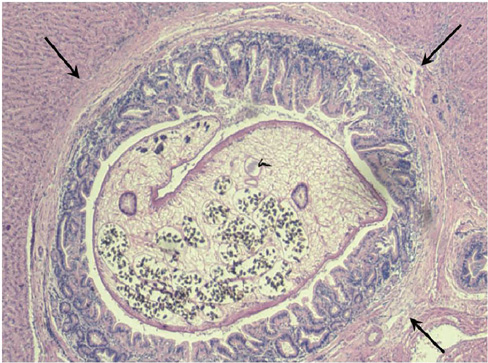
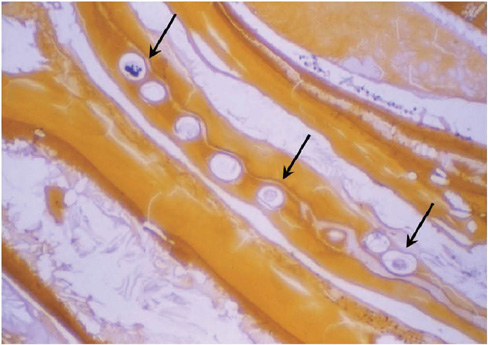
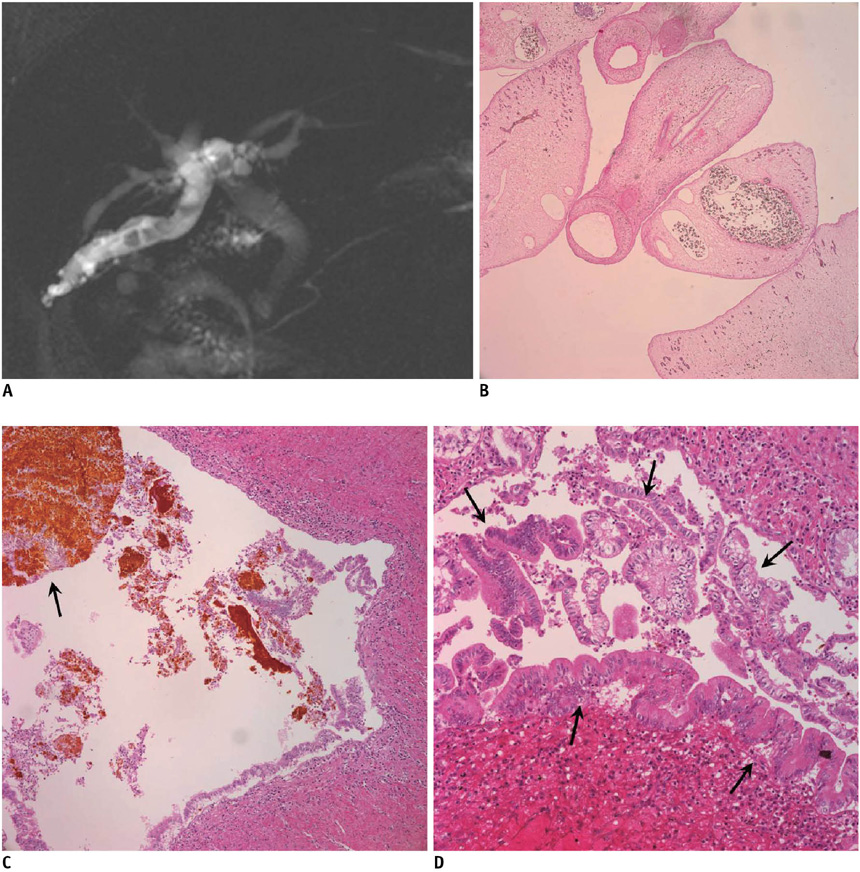
- Liver fluke disease is a chronic parasitic inflammatory disease of the bile ducts. Infection occurs through ingestion of fluke-infested, fresh-water raw fish. The most well-known species that cause human infection are Clonorchis sinensis, Opisthorchis viverrini and Opisthorchis felineus. Adult flukes settle in the small intrahepatic bile ducts and then they live there for 20-30 years. The long-lived flukes cause long-lasting chronic inflammation of the bile ducts and this produces epithelial hyperplasia, periductal fibrosis and bile duct dilatation. The vast majority of patients are asymptomatic, but the patients with heavy infection suffer from lassitude and nonspecific abdominal complaints. The complications are stone formation, recurrent pyogenic cholangitis and cholangiocarcinoma. Approximately 35 million people are infected with liver flukes throughout the world and the exceptionally high incidence of cholangiocarcinoma in some endemic areas is closely related with a high prevalence of liver fluke infection. Considering the impact of this food-borne malady on public health and the severe possible clinical consequences, liver fluke infection should not be forgotten or neglected.
Keyword
MeSH Terms
-
Animals
Bile Duct Neoplasms/*diagnosis/epidemiology/*parasitology
Bile Ducts, Intrahepatic/*parasitology
Biological Markers/analysis
Cholangiocarcinoma/*diagnosis/epidemiology/*parasitology
Cholangitis/diagnosis/parasitology
Clonorchiasis/*complications/*diagnosis/epidemiology/parasitology
Clonorchis sinensis
Humans
Incidence
Opisthorchiasis/*complications/*diagnosis/epidemiology/parasitology
Opisthorchis
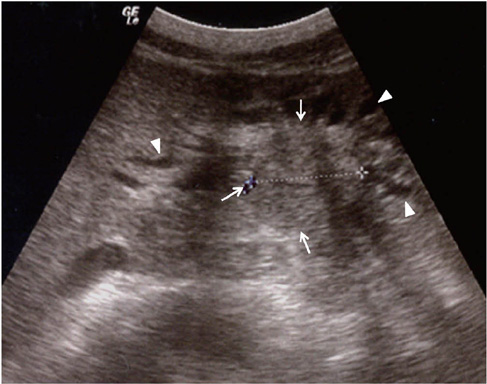
Figure
Cited by 2 articles
-
Survival analysis of extrahepatic cholangiocarcinoma based on surveillance, epidemiology, and end results database
Hassam Ali, Joshua Zweigle, Pratik Patel, Brandon Tedder, Rafeh Khan, Saurabh Agrawal
Ann Hepatobiliary Pancreat Surg. 2023;27(2):151-157. doi: 10.14701/ahbps.22-090.Imaging findings of intrahepatic cholangiocarcinoma for prognosis prediction and treatment decision-making: a narrative review
Jun Gu Kang, Taek Chung, Dong Kyu Kim, Hyungjin Rhee
Ewha Med J. 2024;47(4):e66. doi: 10.12771/emj.2024.e66.
Reference
-
1. Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1995. 849:1–157.2. Rim HJ, Kim KH, Joo KH. Classification and host specificity of Metagonimus spp. from Korean freshwater fish. Korean J Parasitol. 1996. 34:7–14.3. Rim HJ. Clonorchiasis: an update. J Helminthol. 2005. 79:269–281.4. Jongsuksuntigul P, Imsomboon T, Teerarat S, Suruthanavanith P, Kongpradit S. Consequences of opisthorchiasis control programme in northeastern Thailand. J Trop Med Parasitol. 1996. 19:24–39.5. Lim JH. Radiologic findings of clonorchiasis. AJR Am J Roentgenol. 1990. 155:1001–1008.6. Riganti M, Pungpak S, Punpoowong B, Bunnag D, Harinasuta T. Human pathology of Opisthorchis viverrini infection: a comparison of adults and children. Southeast Asian J Trop Med Public Health. 1989. 20:95–100.7. Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003. 88:209–220.8. Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003. 88:221–227.9. Harinasuta T, Riganti M, Bunnag D. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung. 1984. 34:1167–1169.10. Upatham ES, Viyanant V, Kurathong S, Rojborwonwitaya J, Brockelman WY, Ardsungnoen S, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull World Health Organ. 1984. 62:451–461.11. Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005. 5:31–41.12. Coordinating Office of the National Survey on the Important Human Parasitic Diseases. A national survey on current status of the important parasitic diseases in human population. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005. 23:332–340.13. Jongsuksuntigul P, Imsomboon T. Epidemiology of opisthorchiasis and national control program in Thailand. Southeast Asian J Trop Med Public Health. 1998. 29:327–332.14. Kaewpitoon N, Kaewpitoon SJ, Pengsaa P. Opisthorchiasis in Thailand: review and current status. World J Gastroenterol. 2008. 14:2297–2302.15. Upatham ES, Viyanant V, Kurathong S, Brockelman WY, Menaruchi A, Saowakontha S, et al. Morbidity in relation to intensity of infection in Opisthorchiasis viverrini: study of a community in Khon Kaen, Thailand. Am J Trop Med Hyg. 1982. 31:1156–1163.16. Prevalence of intestinal parasitic infection in Korea, the 7th Report. 2004. Korea Association of Health Promotion;122–123.17. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Cholangiocarcinoma and Clonorchis sinensis infection: a case-control study in Korea. J Hepatol. 2006. 44:1066–1073.18. Flavell DJ. Liver-fluke infection as an aetiological factor in bile-duct carcinoma of man. Trans R Soc Trop Med Hyg. 1981. 75:814–824.19. Pae WK. Bacteriological and parasitological study of stone. J Korean Surg Soc. 1968. 10:377–380.20. Joo KR, Bang SJ. A bile based study of Clonorchis sinensis infections in patients with biliary tract diseases in Ulsan, Korea. Yonsei Med J. 2005. 46:794–798.21. Choi D, Lim JH, Lee KT, Lee JK, Choi SH, Heo JS, et al. Gallstones and Clonorchis sinensis infection: a hospital-based case-control study in Korea. J Gastroenterol Hepatol. 2008. 23:e399–e404.22. Wong WT, Teoh-Chan CH, Huang CT, Cheng FC, Ong GB. The bacteriology of recurrent pyogenic cholangitis and associated diseases. J Hyg (Lond). 1981. 87:407–412.23. Nakanuma Y, Sripa B, Vantanasapt V, Leong A-Y, Ponchon T, Ishak KG. Hamilton SR, Aaltonen LA, editors. Intrahepatic cholangiocarcinoma. World Health Organization classification of tumors. Pathology and genetics of tumours of the digestive system. 2000. Lyon, France: IARC Press;173–180.24. Jung KW, Won YJ, Park S, Kong HJ, Sung J, Shin HR, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009. 24:995–1003.25. Bhamarapravati N, Virranuvatti V. Liver diseases in Thailand. An analysis of liver biopsies. Am J Gastroenterol. 1966. 45:267–275.26. Watanapa P, Watanapa WB. Liver fluke-associated cholangiocarcinoma. Br J Surg. 2002. 89:962–970.27. Haswell-Elkins MR, Satarug S, Tsuda M, Mairiang E, Esumi H, Sithithaworn P, et al. Liver fluke infection and cholangiocarcinoma: model of endogenous nitric oxide and extragastric nitrosation in human carcinogenesis. Mutat Res. 1994. 305:241–252.28. Elkins DB, Mairiang E, Sithithaworn P, Mairiang P, Chaiyakum J, Chamadol N, et al. Cross-sectional patterns of hepatobiliary abnormalities and possible precursor conditions of cholangiocarcinoma associated with Opisthorchis viverrini infection in humans. Am J Trop Med Hyg. 1996. 55:295–301.29. Haswell-Elkins MR, Mairiang E, Mairiang P, Chaiyakum J, Chamadol N, Loapaiboon V, et al. Cross-sectional study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994. 59:505–509.30. Sithithaworn P, Haswell-Elkins MR, Mairiang P, Satarug S, Mairiang E, Vatanasapt V, et al. Parasite-associated morbidity: liver fluke infection and bile duct cancer in northeast Thailand. Int J Parasitol. 1994. 24:833–843.31. Kurathong S, Lerdverasirikul P, Wongpaitoon V, Pramoolsinsap C, Kanjanapitak A, Varavithya W, et al. Opisthorchis viverrini infection and cholangiocarcinoma. A prospective, case-controlled study. Gastroenterology. 1985. 89:151–156.32. Elkins DB, Haswell-Elkins MR, Mairiang E, Mairiang P, Sithithaworn P, Kaewkes S, et al. A high frequency of hepatobiliary disease and suspected cholangiocarcinoma associated with heavy Opisthorchis viverrini infection in a small community in north-east Thailand. Trans R Soc Trop Med Hyg. 1990. 84:715–719.33. Chung CS, Lee SK. An epidemiological study of primary liver carcinomas in Pusan area with special reference to clonorchiasis. Korean J Pathol. 1976. 10:33–46.34. Shin HR, Lee CU, Park HJ, Seol SY, Chung JM, Choi HC, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: a case-control study in Pusan, Korea. Int J Epidemiol. 1996. 25:933–940.35. Suh KS, Roh HR, Koh YT, Lee KU, Park YH, Kim SW. Clinicopathologic features of the intraductal growth type of peripheral cholangiocarcinoma. Hepatology. 2000. 31:12–17.36. Jang KT, Hong SM, Lee KT, Lee JG, Choi SH, Heo JS, et al. Intraductal papillary neoplasm of the bile duct associated with Clonorchis sinensis infection. Virchows Arch. 2008. 453:589–598.37. Hou PC. The relationship between primary carcinoma of the liver and infestation with Clonorchis sinensis. J Pathol Bacteriol. 1956. 72:239–246.38. Kim YI. Liver carcinoma and liver fluke infection. Arzneimittelforschung. 1984. 34:1121–1126.39. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.40. Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, et al. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006. 44:350–358.41. Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000. 7:542–550.42. Chen TC, Nakanuma Y, Zen Y, Chen MF, Jan YY, Yeh TS, et al. Intraductal papillary neoplasia of the liver associated with hepatolithiasis. Hepatology. 2001. 34:651–658.43. Nakanuma Y, Sasaki M, Ishikawa A, Tsui W, Chen TC, Huang SF. Biliary papillary neoplasm of the liver. Histol Histopathol. 2002. 17:851–861.44. Shimonishi T, Zen Y, Chen TC, Chen MF, Jan YY, Yeh TS, et al. Increasing expression of gastrointestinal phenotypes and p53 along with histologic progression of intraductal papillary neoplasia of the liver. Hum Pathol. 2002. 33:503–511.45. Lee JH, Yang HM, Bak UB, Rim HJ. Promoting role of Clonorchis sinensis infection on induction of cholangiocarcinoma during two-step carcinogenesis. Korean J Parasitol. 1994. 32:13–18.46. Lee JH, Rim HJ, Bak UB. Effect of Clonorchis sinensis infection and dimethylnitrosamine administration on the induction of cholangiocarcinoma in Syrian golden hamsters. Korean J Parasitol. 1993. 31:21–30.47. Satarug S, Haswell-Elkins MR, Sithithaworn P, Bartsch H, Ohshima H, Tsuda M, et al. Relationships between the synthesis of N-nitrosodimethylamine and immune responses to chronic infection with the carcinogenic parasite, Opisthorchis viverrini, in men. Carcinogenesis. 1998. 19:485–491.48. Thamavit W, Pairojkul C, Tiwawech D, Shirai T, Ito N. Strong promoting effect of Opisthorchis viverrini infection on dimethylnitrosamine-initiated hamster liver. Cancer Lett. 1994. 78:121–125.49. Levy C, Lymp J, Angulo P, Gores GJ, Larusso N, Lindor KD. The value of serum CA 19-9 in predicting cholangiocarcinomas in patients with primary sclerosing cholangitis. Dig Dis Sci. 2005. 50:1734–1740.50. Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000. 95:204–207.51. John AR, Haghighi KS, Taniere P, Esmat ME, Tan YM, Bramhall SR. Is a raised CA 19-9 level diagnostic for a cholangiocarcinoma in patients with no history of sclerosing cholangitis? Dig Surg. 2006. 23:319–324.52. Nehls O, Gregor M, Klump B. Serum and bile markers for cholangiocarcinoma. Semin Liver Dis. 2004. 24:139–154.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chromosomes of the liver fluke, Clonorchis sinensis
- Hepatic Parasitic Abscess Caused by Clonorchiasis: Unusual CT Findings of Clonorchiasis
- Relationship Between the Snails of Lymnaea spp. and the Common Liver Flukes, Fasciola spp.-With Special Reference to the Susceptibility of the Snails to the Liver Flukes
- Liver Flukes and Cholangiocarcinoma: Mechanism of Carcinogenesis
- A Human Case of Hepatic Resection for Liver Fascioliasis in Korea