J Vet Sci.
2011 Jun;12(2):191-193. 10.4142/jvs.2011.12.2.191.
A simplified PCR assay for fast and easy mycoplasma mastitis screening in dairy cattle
- Affiliations
-
- 1Animal Health Laboratory, Faculty of Veterinary Medicine, Rakuno Gakuen University, Hokkaido 069-8501, Japan. higuchi@rakuno.ac.jp
- 2Veterinary Biochemistry, Faculty of Veterinary Medicine, Rakuno Gakuen University, Hokkaido 069-8501, Japan.
- 3Department of Animal Health 1, School of Veterinary Medicine, Azabu University, Kanagawa 229-8501, Japan.
- 4Tokachi Federation of Agricultural Cooperatives, Minami 6-chome, Nishi 14-jo, Hokkaido 080-0024, Japan.
- 5Nanbu Veterinary Clinical Centre, Tokachi NOSAI, 180-1 Tokachi, Hokkaido 089-2106, Japan.
- 6Animal Health Department, Technical Service, Agricultural and Veterinary Division, Meiji Seika Kaisha Ltd., 2-4-6 Kyobashi, Tokyo 104-8002, Japan.
- 7Food Microbiology and Food Safety, Faculty of Veterinary Medicine, Rakuno Gakuen University, Hokkaido 069-8501, Japan.
- KMID: 1106563
- DOI: http://doi.org/10.4142/jvs.2011.12.2.191
Abstract
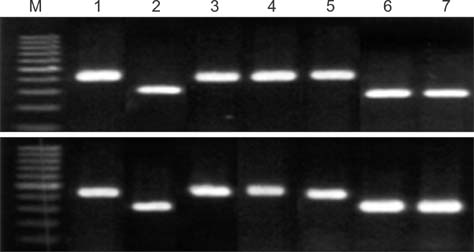
- A simplified polymerase chain reaction (PCR) assay was developed for fast and easy screening of mycoplasma mastitis in dairy cattle. Species of major mycoplasma strains [Mycoplasma (M.) bovis, M. arginini, M. bovigenitalium, M. californicum, M. bovirhinis, M. alkalescens and M. canadense] in cultured milk samples were detected by this simplified PCR-based method as well as a standard PCR technique. The minimum concentration limit for detecting mycoplasma by the simplified PCR was estimated to be about 2.5 x 10(3) cfu/mL and was similar to that of the standard PCR. We compared the specificity and sensitivity of the simplified PCR to those of a culture method. Out of 1,685 milk samples cultured in mycoplasma broth, the simplified PCR detected Mycoplasma DNA in 152 that were also positive according to the culture assay. The sensitivity and specificity of the simplified PCR were 98.7% and 99.7%, respectively, for detecting mycoplasma in those cultures. The results obtained by the simplified PCR were consistent with ones from standard PCR. This newly developed simplified PCR, which does not require DNA purification, can analyze about 300 cultured samples within 3 h. The results from our study suggest that the simplified PCR can be used for mycoplasma mastitis screening in large-scale dairy farms.
Keyword
MeSH Terms
-
Animals
Cattle
Colony Count, Microbial/veterinary
DNA, Bacterial/chemistry/genetics
Disease Outbreaks/prevention & control/veterinary
Female
Mastitis, Bovine/diagnosis/*microbiology
Milk/cytology/*microbiology
Mycoplasma/genetics/*isolation & purification
Mycoplasma Infections/diagnosis/microbiology/*veterinary
Polymerase Chain Reaction/veterinary
Figure
Reference
-
1. Alshahni MM, Makimura K, Yamada T, Satoh K, Ishihara Y, Takatori K, Sawada T. Direct colony PCR of several medically important fungi using Ampdirect plus. Jpn J Infect Dis. 2009. 62:164–167.2. Baird SC, Carman J, Dinsmore RP, Walker RL, Collins JK. Detection and identification of Mycoplasma from bovine mastitis infections using a nested polymerase chain reaction. J Vet Diagn Invest. 1999. 11:432–435.
Article3. Bushnell RB. Mycoplasma mastitis. Vet Clin North Am Large Anim Pract. 1984. 6:301–312.
Article4. Ghadersohi A, Coelen RJ, Hirst RG. Development of a specific DNA probe and PCR for the detection of Mycoplasma bovis. Vet Microbiol. 1997. 56:87–98.
Article5. Jasper DE. The role of Mycoplasma in bovine mastitis. J Am Vet Med Assoc. 1982. 181:158–162.6. Kirk JH, Lauerman LH. Mycoplasma mastitis in dairy cows. Compend Contin Educ Pract Vet. 1994. 16:541–551.7. Nicholas RAJ, Ayling RD. Mycoplasma bovis: disease, diagnosis, and control. Res Vet Sci. 2003. 74:105–112.8. Pfützner H, Sachse K. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev Sci Tech. 1996. 15:1477–1494.9. Riffon R, Sayasith K, Khalil H, Dubreuil P, Drolet M, Lagacé J. Development of a rapid and sensitive test for identification of major pathogens in bovine mastitis by PCR. J Clin Microbiol. 2001. 39:2584–2589.
Article10. Sung H, Kang SH, Bae YJ, Hong JT, Chung YB, Lee CK, Song S. PCR-based detection of Mycoplasma species. J Microbiol. 2006. 44:42–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence and molecular characterization of Cryptosporidium spp. in dairy cattle from farms in China
- Epizootiological and Bacteriological Studies of Bovine Tuberculosis in Korea -Report 2 A Study on the Acid-Fast Bacteria Isolated from Tuberculin Reactors of Dairy Cattle in 1971
- Diagnosis of Mycoplasma hyorhinis infection in pigs by PCR amplification of 16S-23S rRNA internal transcribed spacer region
- A sensitive and specific polymerase chain reaction to detect Mycoplasma hyopnemoniae using Mycoplasma protein P97 gene
- Molecular characteristics and antimicrobial susceptibility profiles of bovine mastitis agents in western Türkiye