J Vet Sci.
2007 Mar;8(1):15-20. 10.4142/jvs.2007.8.1.15.
Organotypic slice culture of the hypothalamic paraventricular nucleus of rat
- Affiliations
-
- 1Laboratory of Veterinary Pharmacology, BK21 Program for Veterinary Science and College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. pandryu@plaza.snu.ac.kr
- 2Department of Physiology, Institute of Health Science and Medical Research Center for Neural Dysfunction, Gyeongsang National University College of Medicine, Jinju 660-701, Korea.
- KMID: 1104231
- DOI: http://doi.org/10.4142/jvs.2007.8.1.15
Abstract
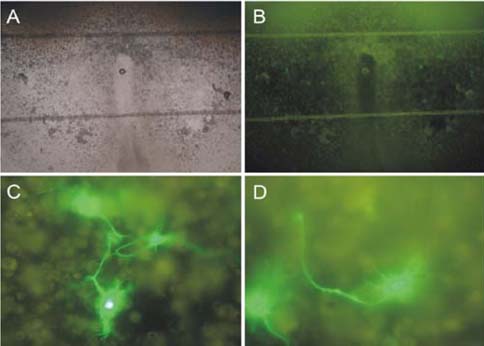
- Organotypic slice cultures have been developed as an alternative to acute brain slices because the neuronal viability and synaptic connectivity in these cultures can be preserved well for a prolonged period of time. This study evaluated a stationary organotypic slice culture developed for the hypothalamic paraventricular nucleus (PVN) of rat. The results showed that the slice cultures maintain the typical shape of the nucleus, the immunocytochemical signals for oxytocin, vasopressin, and corticotropin-releasing hormone, and the electrophysiological properties of PVN neurons for up to 3 weeks in vitro. The PVN neurons in the culture expressed the green fluorescent protein gene that had been delivered by the adenoviral vectors. The results indicate that the cultured slices preserve the properties of the PVN neurons, and can be used in longterm studies on these neurons in vitro.
Keyword
MeSH Terms
-
Adenoviridae
Animals
Cell Culture Techniques/*methods
Corticotropin-Releasing Hormone/metabolism
Electrophysiology
Genetic Vectors
Green Fluorescent Proteins/metabolism
Immunohistochemistry
Neurons/*cytology/metabolism
Oxazines
Oxytocin/metabolism
Paraventricular Hypothalamic Nucleus/*anatomy & histology/cytology/metabolism
Rats
Vasopressins/metabolism
Figure
Reference
-
1. Arima H, House SB, Gainer H, Aguilera G. Neuronal activity is required for the circadian rhythm of vasopressin gene transcription in the suprachiasmatic nucleus in vitro. Endocrinology. 2002. 143:4165–4171.
Article2. Armstrong WE. Paxinos G, editor. Hypothalamic supraoptic and paraventricular nuclei. The Rat Nervous System. 2004. 3rd. San Diego: Elsevier Academic Press;369–388.
Article3. Bartanusz V, Muller D, Gaillard RC, Streit P, Vutskits L, Kiss JZ. Local gamma-aminobutyric acid and glutamate circuit control of hypophyseotrophic corticotropin-releasing factor neuron activity in the paraventricular nucleus of the hypothalamus. Eur J Neurosci. 2004. 19:777–782.
Article4. Belenky M, Wagner S, Yarom Y, Matzner H, Cohen S, Castel M. The suprachiasmatic nucleus in stationary organotypic culture. Neuroscience. 1996. 70:127–143.
Article5. Benediktsson AM, Schachtele SJ, Green SH, Dailey ME. Ballistic labeling and dynamic imaging of astrocytes in organotypic hippocampal slice cultures. J Neurosci Methods. 2005. 141:41–53.
Article6. Bergold PJ, Casaccia-Bonnefil P. Preparation of organotypic hippocampal slice cultures using the membrane filter method. Methods Mol Biol. 1997. 72:15–22.
Article7. Bertini LT, Kursner C, Gaillard RC, Corder R, Kiss JZ. A tissue culture model of the hypophysiotrophic CRH producing neuronal system. Neuroendocrinology. 1993. 57:716–728.
Article8. Chong W, Li LH, Lee K, Lee MH, Park JB, Ryu PD. Subtypes of α1- and α2-adrenoceptors mediating noradrenergic modulation of spontaneous inhibitory postsynaptic currents in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2004. 16:450–457.
Article9. Fields RL, House SB, Gainer H. Regulatory domains in the intergenic region of the oxytocin and vasopressin genes that control their hypothalamus-specific expression in vitro. J Neurosci. 2003. 23:7801–7809.
Article10. Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981. 4:329–342.
Article11. Gähwiler BH, Capogna M, Debanne D, McKinney RA, Thompson SM. Organotypic slice cultures: a technique has come of age. Trends Neurosci. 1997. 20:471–477.
Article12. Graulich J, Hoffmann U, Maier RF, Ruscher K, Pomper JK, Ko HK, Gabriel S, Obladen M, Heinemann U. Acute neuronal injury after hypoxia is influenced by the reoxygenation mode in juvenile hippocampal slice cultures. Brain Res Dev Brain Res. 2002. 137:35–42.
Article13. Han SK, Chong W, Li LH, Lee IS, Murase K, Ryu PD. Noradrenaline excites and inhibits GABAergic transmission in parvocellular neurons of rat hypothalamic paraventricular nucleus. J Neurophysiol. 2002. 87:2287–2296.
Article14. Hilton KJ, Bateson AN, King AE. A model of organotypic rat spinal slice culture and bolistic transfection to elucidate factors that drive the preprotachykinin-A promoter. Brain Res Brain Res Rev. 2004. 46:191–203.
Article15. House SB, Thomas A, Kusano K, Gainer H. Stationary organotypic cultures of oxytocin and vasopressin magnocellular neurones from rat and mouse hypothalamus. J Neuroendocrinol. 1998. 10:849–861.
Article16. Keir SD, House SB, Li J, Xiao X, Gainer H. Gene transfer into hypothalamic organotypic cultures using an adeno-associated virus vector. Exp Neurol. 1999. 160:313–316.
Article17. Kusano K, House SB, Gainer H. Effects of osmotic pressure and brain-derived neurotrophic factor on the survival of postnatal hypothalamic oxytocinergic and vasopressinergic neurons in dissociated cell culture. J Neuroendocrinol. 1999. 11:145–152.
Article18. Kuwahara S, Arima H, Banno R, Sato I, Kondo N, Oiso Y. Regulation of vasopressin gene expression by cAMP and glucocorticoids in parvocellular neurons of the paraventricular nucleus in rat hypothalamic organotypic cultures. J Neurosci. 2003. 23:10231–10237.
Article19. Leutgeb JK, Frey JU, Behnisch T. LTP in cultured hippocampal-entorhinal cortex slices from young adult (P25-30) rat. J Neurosci Methods. 2003. 130:19–32.
Article20. Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991. 37:173–182.
Article21. Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983. 6:269–324.
Article22. Tasker JG, Dudek FE. Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol. 1991. 434:271–293.
Article23. Ueta Y, Fujihara H, Serino R, Dayanithi G, Ozawa H, Matsuda K, Kawata M, Yamada J, Ueno S, Fukuda A, Murphy D. Transgenic expression of enhanced green fluorescent protein enables direct visualization for physiological studies of vasopressin neurons and isolated nerve terminals of the rat. Endocrinology. 2005. 146:406–413.
Article24. Walker SJ, Vrana KE. Pituitary corticotroph function during the stress hyporesponsive period in neonatal rats. Neuroendocrinology. 1993. 57:1003–1010.
Article25. Xiang Z, Hrabetova S, Moskowitz SI, Casaccia-Bonnefil P, Young SR, Nimmrich VC, Tiedge H, Einheber S, Karnup S, Bianchi R, Bergold PJ. Long-term maintenance of mature hippocampal slices in vitro. J Neurosci Methods. 2000. 98:145–154.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Chronic Alcohol Intake on Vasopressin and Oxytocin-containing Neurons in the Paraventricular and Supraoptic Nucleus of the Rat Hypothalamus
- Immunohistochemical Localization of Brain Natriuretic Peptide in the Hypothalamus of the Rat
- Coexistence of Tyrosine Hydroxylase and Nicotinamide Adenine Dinucleotide Phosphate-Diaphorase in Hypothalamic Neurons of the Rat
- Dopaminergic Neurons in the Diencephalon of Striped Field Mouse[Apodemus agrarius coreae]
- Postnatal Development of Brain Natriuretic Peptide-immunoreactive Neuron in the Hypothalamus of the Rat