Korean J Radiol.
2009 Dec;10(6):635-640. 10.3348/kjr.2009.10.6.635.
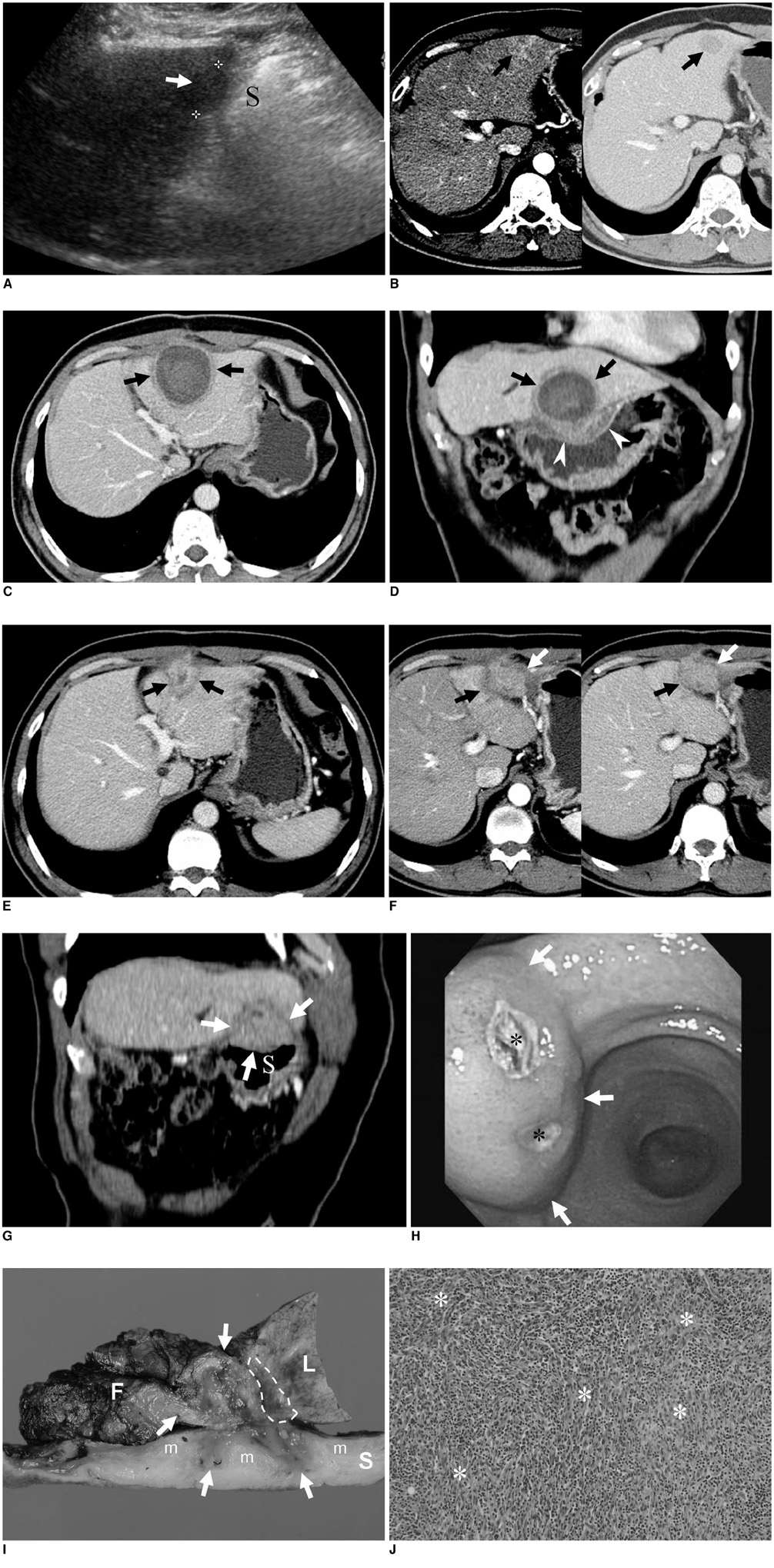
Inflammatory Myofibroblastic Tumor: a Possible Complication of Percutaneous Radiofrequency Ablation for Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Radiology, Seoul National University Hospital, Korea.
- 2Institute of Radiation Medicine, Seoul National University Hospital, Korea.shkim@radcom.snu.ac.kr
- 3Department of Pathology, Seoul National University Hospital, Korea.
- KMID: 1102568
- DOI: http://doi.org/10.3348/kjr.2009.10.6.635
Abstract
- An inflammatory myofibroblastic tumor (IMT) is an uncommon, benign lesion characterized by the mesenchymal proliferation and infiltration of inflammatory cells composed primarily of lymphocytes and plasma cells. A percutaneous radiofrequency ablation (RFA) is an effective and safe therapeutic modality used for the management of liver malignancies. Here we report, for the first time, a case of IMT as a complication of RFA for hepatocellular carcinoma in a 61-year-old man with a Child's class A hepatitis B-related liver cirrhosis. Gastrohepatic fistula formation was pathologically proven and associated with the RFA. Such a longstanding inflammation of the fistula might have been a possible cause of the development of IMT in this case.
MeSH Terms
Figure
Reference
-
1. Anthony PP, Telesinghe PU. Inflammatory pseudotumour of the liver. J Clin Pathol. 1986. 39:761–768.2. Lawrence B, Perez-Atayde A, Hibbard MK, Rubin BP, Dal Cin P, Pinkus JL, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000. 157:377–384.3. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Ierace T, Solbiati L, et al. Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology. 2000. 214:761–768.4. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003. 23:123–134.5. Bruix J, Sherman M. Practice Guidelines Committee. American Association for the Study of Liver Diseases. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005. 42:1208–1236.6. Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007. 42:163–171.7. Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepatocellular carcinoma: imaging spectrum and management. Radiographics. 2005. 25:S57–S68.8. Gleason BC, Hornick JL. Inflammatory myofibroblastic tumours: where are we now? J Clin Pathol. 2008. 61:428–437.9. Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995. 19:859–872.10. Bessoud B, Doenz F, Qanadli SD, Nordback P, Schnyder P, Denys A. Enterobiliary fistula after radiofrequency ablation of liver metastases. J Vasc Interv Radiol. 2003. 14:1581–1584.11. Falco A, Orlando D, Sciarra R, Sergiacomo L. A case of biliary gastric fistula following percutaneous radiofrequency thermal ablation of hepatocellular carcinoma. World J Gastroenterol. 2007. 13:804–805.12. Kondo Y, Yoshida H, Shiina S, Tateishi R, Teratani T, Omata M. Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg. 2006. 93:1277–1282.13. Kim HC, Lee JM, Kim KW, Park SH, Kim SH, Lee JY, et al. Gastrointestinal stromal tumors of the stomach: CT findings and prediction of malignancy. AJR Am J Roentgenol. 2004. 183:893–898.14. Lokken RP, Gervais DA, Arellano RS, Tuncali K, Morrison PR, Tatli S, et al. Inflammatory nodules mimic applicator track seeding after percutaneous ablation of renal tumors. AJR Am J Roentgenol. 2007. 189:845–848.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Small Bowel Perforation after Percutaneous Ultrasound-guided Radiofrequency Ablation of Hepatocellular Carcinoma
- Completely Ablated Hepatocellular Carcinoma by Percutaneous Radiofrequency Thermal Ablation
- Microwave thermosphere versus radiofrequency ablation for hepatocellular carcinoma: Are we approaching the time to end the debate?
- Chemoembolization combined with radiofrequency ablation is the best option for the local treatment of early hepatocellular carcinoma?
- Radiofrequency Ablation of Hepatic Cysts: Case Report