J Vet Sci.
2011 Mar;12(1):7-13. 10.4142/jvs.2011.12.1.7.
Expression patterns of influenza virus receptors in the respiratory tracts of four species of poultry
- Affiliations
-
- 1Laboratory of Veterinary Anatomy, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea. ssnahm@konkuk.ac.kr
- 2Avian Diseases Laboratory, College of Veterinary Medicine, Konkuk University, Seoul 143-701, Korea.
- KMID: 1067331
- DOI: http://doi.org/10.4142/jvs.2011.12.1.7
Abstract
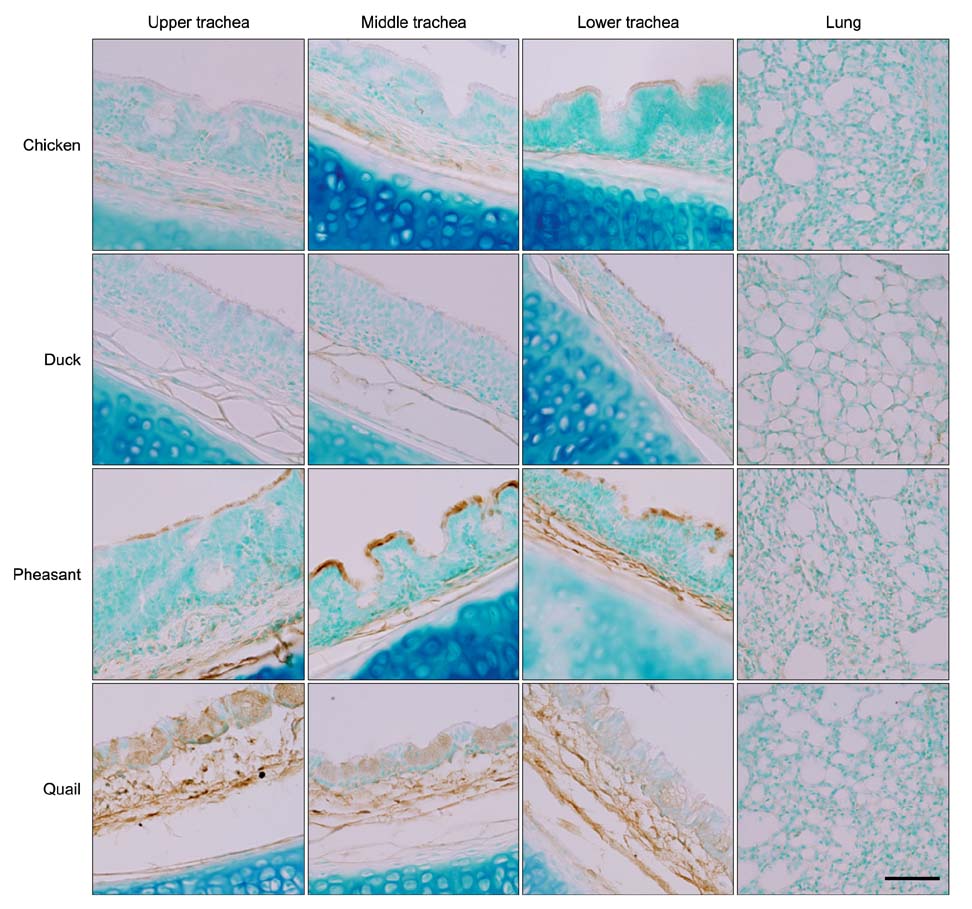
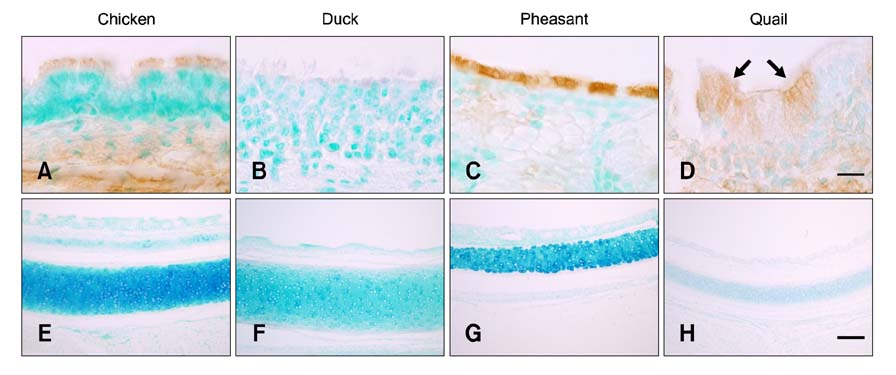
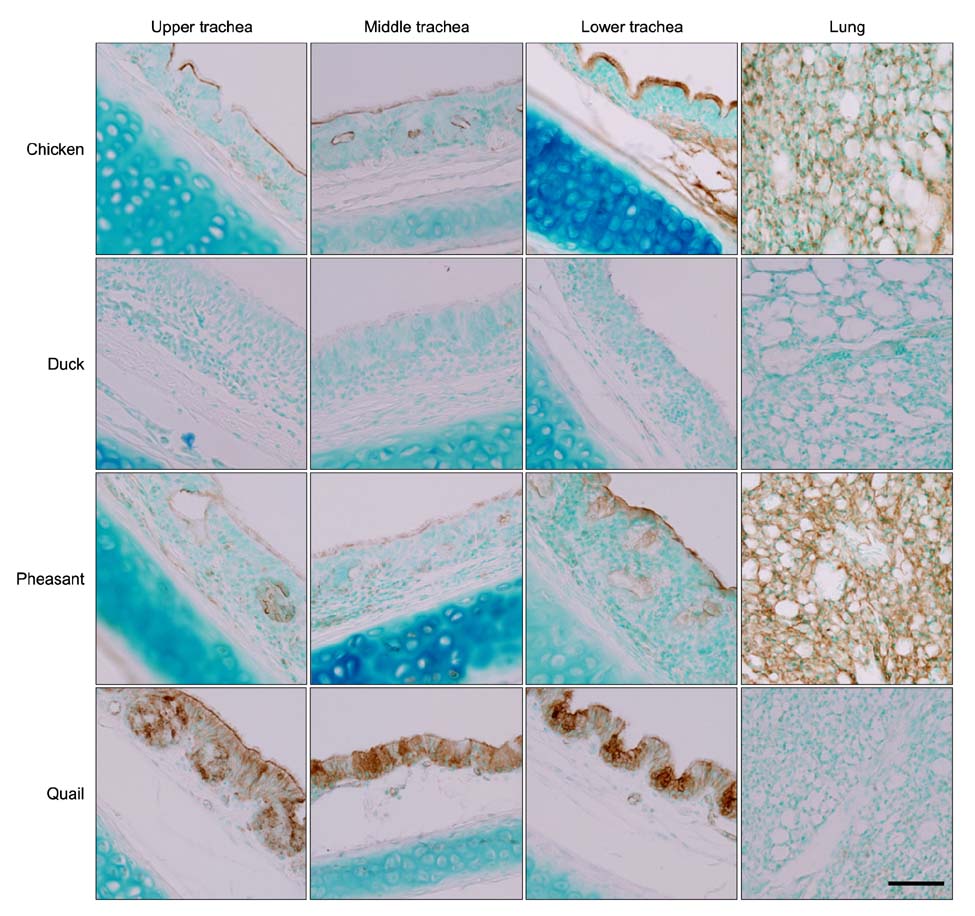
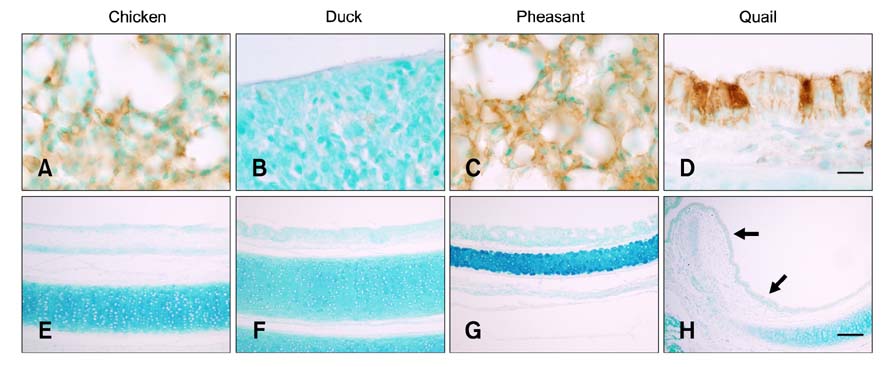
- The primary determinant of influenza virus infectivity is the type of linkage between sialic acid and oligosaccharides on the host cells. Hemagglutinin of avian influenza viruses preferentially binds to sialic acids linked to galactose by an alpha-2,3 linkage whereas hemagglutinin of human influenza viruses binds to sialic acids with an alpha-2,6 linkage. The distribution patterns of influenza receptors in the avian respiratory tracts are of particular interest because these are important for initial viral attachment, replication, and transmission to other species. In this study, we examined the distribution patterns of influenza receptors in the respiratory tract of chickens, ducks, pheasants, and quails because these species have been known to act as intermediate hosts in interspecies transmission. Lectin histochemistry was performed to detect receptor-bearing cells. Cell-specific distribution of the receptors was determined and expression densities were compared. We observed species-, site-, and cell-specific variations in receptor expression. In general, receptor expression was the highest in quails and lowest in ducks. Pheasants and quails had abundant expression of both types of receptors throughout the respiratory tract. These results indicate that pheasants and quails may play important roles as intermediate hosts for the generation of influenza viruses with pandemic potential.
MeSH Terms
-
Animals
Cell Membrane/metabolism/virology
Hemagglutinin Glycoproteins, Influenza Virus/metabolism
Host-Pathogen Interactions
Influenza A virus/*metabolism
Influenza in Birds/metabolism/transmission
Lectins/metabolism
Poultry/metabolism/*virology
Poultry Diseases/metabolism
Receptors, Cell Surface/analysis/chemistry/metabolism
Receptors, Virus/*analysis/metabolism
Respiratory System/*chemistry
Sialic Acids/metabolism
Species Specificity
Specific Pathogen-Free Organisms
Figure
Reference
-
1. Campitelli L, Fabiani C, Puzelli S, Fioretti A, Foni E, De Marco A, Krauss S, Webster RG, Donatelli I. H3N2 influenza viruses from domestic chickens in Italy: an increasing role for chickens in the ecology of influenza? J Gen Virol. 2002. 83:413–420.
Article2. Campitelli L, Mogavero E, De Marco MA, Delogu M, Puzelli S, Frezza F, Facchini M, Chiapponi C, Foni E, Cordioli P, Webby R, Barigazzi G, Webster RG, Donatelli I. Interspecies transmission of an H7N3 influenza virus from wild birds to intensively reared domestic poultry in Italy. Virology. 2004. 323:24–36.
Article3. Ellström P, Jourdain E, Gunnarsson O, Waldenström J, Olsen B. The "human influenza receptor" Neu5Acα2,6Gal is expressed among different taxa of wild birds. Arch Virol. 2009. 154:1533–1537.
Article4. Gambaryan A, Webster R, Matrosovich M. Differences between influenza virus receptors on target cells of duck and chicken. Arch Virol. 2002. 147:1197–1208.
Article5. Humberd J, Guan Y, Webster RG. Comparison of the replication of influenza A viruses in Chinese ring-necked pheasants and Chukar partridges. J Virol. 2006. 80:2151–2161.
Article6. Humberd J, Boyd K, Webster RG. Emergence of influenza A virus variants after prolonged shedding from pheasants. J Virol. 2007. 81:4044–4051.
Article7. Ito T, Couceiro JNSS, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998. 72:7367–7373.
Article8. Konami Y, Yamamoto K, Osawa T, Irimura T. Strong affinity of Maackia amurensis hemagglutinin (MAH) for sialic acid-containing Ser/Thr-linked carbohydrate chains of N-terminal octapeptides from human glycophorin A. FEBS Lett. 1994. 342:334–338.
Article9. Kuchipudi SV, Nelli R, White GA, Bain M, Chang KC, Dunham S. Differences in influenza virus receptors in chickens and ducks: implications for interspecies transmission. J Mol Genet Med. 2009. 3:143–151.
Article10. Lee CW, Saif YM. Avian influenza virus. Comp Immunol Microbiol Infect Dis. 2009. 32:301–310.
Article11. Liu M, He S, Walker D, Zhou N, Perez DR, Mo B, Li F, Huang X, Webster RG, Webby RJ. The influenza virus gene pool in a poultry market in South Central China. Virology. 2003. 305:267–275.
Article12. Nicholls JM, Chan RWY, Russell RJ, Air GM, Peiris JSM. Evolving complexities of influenza virus and its receptors. Trends Microbiol. 2008. 16:149–157.
Article13. Pillai SPS, Lee CW. Species and age related differences in the type and distribution of influenza virus receptors in different tissues of chickens, ducks and turkeys. Virol J. 2010. 7:5.
Article14. Song D, Kang B, Lee C, Jung K, Ha G, Kang D, Park S, Park B, Oh J. Transmission of avian influenza virus (H3N2) to dogs. Emerg Infect Dis. 2008. 14:741–746.
Article15. Wan H, Perez DR. Quail carry sialic acid receptors compatible with binding of avian and human influenza viruses. Virology. 2006. 346:278–286.
Article16. Yao L, Korteweg C, Hsueh W, Gu J. Avian influenza receptor expression in H5N1-infected and noninfected human tissues. FASEB J. 2008. 22:733–740.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influenza Vaccine
- Challenges of influenza A viruses in humans and animals and current animal vaccines as an effective control measure
- Benign Acute Childhood Myositis Associated with Influenza B Virus
- Recovery of respiratory syncytial virus, adenovirus, influenza virus , and parainfluenza virus from nasopharyngeal aspirates from children with acute respiratory tract infections
- Pandemic Threat Posed by Avian Influenza A Viruses