Cardiovasc Prev Pharmacother.
2024 Apr;6(2):65-73. 10.36011/cpp.2024.6.e9.
Cardiovascular risk assessment of newborns in Nigeria using the atherogenic index of plasma and its associations with gestational age and birth weight: a cross-sectional hospital-based study
- Affiliations
-
- 1Department of Paediatrics, Federal Medical Centre, Umuahia, Nigeria
- 2Department of Chemical Pathology, Federal Medical Centre, Umuahia, Nigeria
- 3Department of Paediatrics, University of Nigeria Teaching Hospital (UNTH), University of Nigeria College of Medicine, Enugu, Nigeria
- 4Department of Mental Health, Faculty of Medicine, Nnamdi Azikiwe University, Awka, Nigeria
- KMID: 2554783
- DOI: http://doi.org/10.36011/cpp.2024.6.e9
Abstract
- Background
The prevalence of atherosclerotic cardiovascular disease is rising, and its onset from childhood is widely studied. Prematurity and low birth weight were associated with higher atherogenic risk when assessed using some lipid ratios. However, the atherogenic index of plasma (AIP), a sensitive marker for atherosclerosis is understudied in newborns. Utilizing AIP, this study aimed to determine atherogenic risk prevalence among newborns and its association with gestational age and birth weight.
Methods
Newborns were consecutively recruited, and their lipid profiles were determined. The AIP was calculated as the logarithm to base 10 (log10) of the ratio of molar concentrations of triglyceride to high-density lipoprotein cholesterol. The atherogenic risk was operationalized using AIP: high, >0.24; medium, 0.1–0.24; and low/no risk, <0.1. The relationship between AIP values, gestational age, and birth weight was analyzed using Pearson correlation.
Results
The mean AIP of the 167 newborns studied was –0.35±0.34, which is within the global reference range. Three (1.8%), 10 (6.0%), and 154 (92.2%) newborns were in the high, medium, and low/no atherogenic risk categories, respectively. Hence, 13 newborns (7.8%) had medium to high atherogenic risk. AIP had a moderate significantly positive relationship only with gestational age (r=0.35, P<0.001).
Conclusions
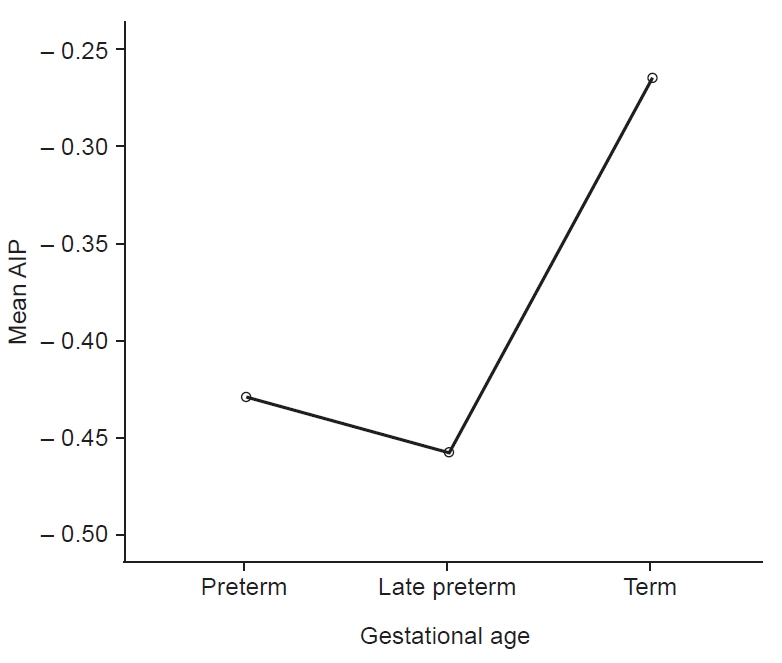
The study found an atherogenic risk prevalence of 7.8% using AIP in newborns which, contrary to previous studies that used other ratios, has no significant association with birth weight, correlating positively with gestational age, though is lowest in late preterms. Follow-up studies will elucidate these findings.
Keyword
Figure
Reference
-
1. Gaziano TA, Bitton A, Anand S, Abrahams-Gessel S, Murphy A. Growing epidemic of coronary heart disease in low- and middle-income countries. Curr Probl Cardiol. 2010; 35:72–115.
Article2. Onen CL. Epidemiology of ischaemic heart disease in sub-Saharan Africa. Cardiovasc J Afr. 2013; 24:34–42.3. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. IHME; 2018.4. Kenchappa Y, Behera N. Assay of neonatal cord blood lipid levels and its correlation with neonatal gestational age, gender and birth weight: a single center experience. Int J Contemp Pediatr. 2016; 3:718–24.
Article5. Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995; 311:171–4.
Article6. Rohrs JH, Schatz D, Winter WE, Davis V. Pediatric lipid disorders in clinical practice treatment and management [Internet]. Medscape; [updated 2023 Apr 20; cited 2024 Feb 5]. Available from: https://emedicine.medscape.com/article/1825087-treatment.7. Concheiro-Guisan A, Sousa-Rouco C, Fernandez-Santamarina I, Gonzalez-Carrero J. Intrauterine myocardial infarction: unsuspected diagnosis in the delivery room. Fetal Pediatr Pathol. 2006; 25:179–84.
Article8. Bezold LI, Pflieger K. Myocardial infarction in childhood [Internet]. Medscape; [updated 2020 Dec 7; cited 2022 Feb 22]. Available from: https://emedicine.medscape.com/article/897453-overview#a3.9. Miyamoto K, Tsuboi T, Suzumura H, Arisaka O. Relationship between aortic intima-media thickening, serum IGF-I and low-density lipoprotein particle diameter in newborns with intrauterine growth restriction. Clin Pediatr Endocrinol. 2009; 18:55–64.
Article10. Stroescu R, Micle I, Marginean O, Bizerea T, Marazan M, Puiu M, et al. Is small for gestational age status associated with an increased risk of atherogenesis? Maedica (Bucur). 2013; 8:315–20.11. Dahlui M, Azahar N, Oche OM, Aziz NA. Risk factors for low birth weight in Nigeria: evidence from the 2013 Nigeria Demographic and Health Survey. Glob Health Action. 2016; 9:28822.
Article12. Awoleke JO. Maternal risk factors for low birth weight babies in Lagos, Nigeria. Arch Gynecol Obstet. 2012; 285:1–6.
Article13. Umeigbo BC, Modebe IA, Iloghalu IC, Eleje GU, Okoro CC, Umeononihu OS, et al. Outcomes of preterm labor and preterm births: a retrospective cross-sectional analytical study in a Nigerian single center population. Obstet Gynecol Res. 2020; 3:17–28.
Article14. Fang J, Zhang JP, Luo CX, Yu XM, Lv LQ. Carotid intima-media thickness in childhood and adolescent obesity relations to abdominal obesity, high triglyceride level and insulin resistance. Int J Med Sci. 2010; 7:278–83.
Article15. Kaneva AM, Potolitsyna NN, Bojko ER, Odland JO. The apolipoprotein B/apolipoprotein A-I ratio as a potential marker of plasma atherogenicity. Dis Markers. 2015; 2015:591454.
Article16. Nimmanapalli HD, Kasi AD, Devapatla PK, Nuttakki V. Lipid ratios, atherogenic coefficient and atherogenic index of plasma as parameters in assessing cardiovascular risk in type 2 diabetes mellitus. Int J Res Med Sci. 2016; 4:2863–9.
Article17. Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Atherogenic lipid profile of Brazilian near-term newborns. Braz J Med Biol Res. 2005; 38:755–60.
Article18. Kharb S, Kaur A, Nanda S. Comparison of cord blood atherogenic index in males and females. Int Cardiovasc Res J. 2010; 4:35–8.19. Niroumand S, Khajedaluee M, Khadem-Rezaiyan M, Abrishami M, Juya M, Khodaee G, et al. Atherogenic index of plasma (AIP): a marker of cardiovascular disease. Med J Islam Repub Iran. 2015; 29:240.20. Tan MH, Johns D, Glazer NB. Pioglitazone reduces atherogenic index of plasma in patients with type 2 diabetes. Clin Chem. 2004; 50:1184–8.
Article21. Dobiasova M. AIP: atherogenic index of plasma as a significant predictor of cardiovascular risk: from research to practice. Vnitr Lek. 2006; 52:64–71.22. Umar LW, Aliyu IS, Akuyam SA. Evaluation of lipid profile in cord blood of full-term Nigerian newborn infants. Sub Saharan Afr J Med. 2017; 4:9–14.
Article23. Armson BA, Allan DS, Casper RF. Umbilical cord blood: counselling, collection, and banking. J Obstet Gynaecol Can. 2015; 37:832–44.
Article24. Williams LA, Wilson DP. Nutritional management of pediatric dyslipidemia. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. MDText.com Inc; [updated 2023 Apr 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK395582/.25. Neal WA, John CC. Disorders of lipoprotein metabolism and transport. In : Kliegman RM, Stanton BF, St. Geme JW, Schor NF, editors. Nelson textbook of pediatrics. 20th ed. Elsevier;2016. p. 691–705.26. Obaji OV, Chukwudi NK, Adiele DK, Adizua UC, Onu JU. Cord blood lipid profile of term and preterm newborns in a tertiary hospital in South East Nigeria: relationship with gestational age and birth weight. Niger Health J. 2023; 23:478–88.27. Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesha. Niger J Paediatr. 1985; 12:111–7.28. Ezenwosu OU, Emodi IJ, Ikefuna AN, Chukwu BF, Osuorah CD. Determinants of academic performance in children with sickle cell anaemia. BMC Pediatr. 2013; 13:189.
Article29. Yashodha HT, Anjum SK. Cord blood lipid profile in late preterm and term neonates. Int J Contemp Pediatr. 2018; 5:542–6.
Article30. Ghiasi A, Ziaei S, Faghihzadeh S. The relationship between levels of lipids and lipoprotein B-100 in maternal serum and umbilical cord serum and assessing their effects on newborn infants anthropometric indices. J Midwifery Reprod Health. 2014; 2:227–32.31. Farinde A. Lipid-lowering agents [Internet]. Medscape; [updated 2023 Apr 4; cited 2024 Feb 5]. Available from: https://emedicine.medscape.com/article/2172172-overview.32. Moll J. Medications that cause high cholesterol levels [Internet]. Verywell Health; [updated 2023 Jun 19; cited 2024 Feb 5]. Available from: https://www.verywell.com/which-drugs-can-raise-cholesterol-levels-698229.33. Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, Yu S, et al. Changes in high-density lipoprotein cholesterol and risks of cardiovascular events: a post hoc analysis from the PICASSO Trial. J Stroke. 2020; 22:108–18.
Article34. Yonezawa R, Okada T, Kitamura T, Fujita H, Inami I, Makimoto M, et al. Very low-density lipoprotein in the cord blood of preterm neonates. Metabolism. 2009; 58:704–7.
Article35. Shoji H, Murano Y, Mori M, Matsunaga N, Ohkawa N, Suganuma H, et al. Lipid profile and atherogenic indices soon after birth in Japanese preterm infants. Acta Paediatr. 2014; 103:22–6.36. Parker CR Jr, Carr BR, Simpson ER, MacDonald PC. Decline in the concentration of low-density lipoprotein-cholesterol in human fetal plasma near term. Metabolism. 1983; 32:919–23.
Article37. Pecks U, Mohaupt MG, Hutten MC, Maass N, Rath W, Escher G. Cholesterol acceptor capacity is preserved by different mechanisms in preterm and term fetuses. Biochim Biophys Acta. 2014; 1841:251–8.
Article38. Aletayeb SM, Dehdashtian M, Aminzadeh M, Moghaddam AR, Mortazavi M, Malamiri RA, et al. Correlation between umbilical cord blood lipid profile and neonatal birth weight. Pediatr Pol. 2013; 88:521–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cord Blood Adiponectin Concentrations in Relation to Newborn Birth Weight, Length and Gender
- Estimation of Fetal Growth by Measurement of Birth Weight for Gestational Age in Newborn
- Changes in the birth weight of full term newborns in Korean population over the past 5 years
- The influence of maternal weight gain to birth weight
- Study on Mean Birth Weight and Medical Care System at City Hospital