Intest Res.
2024 Apr;22(2):172-185. 10.5217/ir.2023.00043.
Efficacy and safety of mirikizumab as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a subgroup analysis of the global phase 3 LUCENT-1 and LUCENT-2 studies
- Affiliations
-
- 1Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- 2Division of Gastroenterology and Hepatology, Department of Internal Medicine, Toho University Sakura Medical Center, Sakura, Japan
- 3Advanced Research Institute, Tokyo Medical and Dental University, Tokyo, Japan
- 4Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Mitaka, Japan
- 5Department of Gastroenterology and Medicine, Fukuoka University Faculty of Medicine, Fukuoka, Japan
- 6Eli Lilly and Company, Indianapolis, IN, USA
- 7Eli Lilly Japan K.K., Kobe, Japan
- KMID: 2554661
- DOI: http://doi.org/10.5217/ir.2023.00043
Abstract
- Background/Aims
Mirikizumab is a p19-directed anti-interleukin-23 antibody with potential efficacy against ulcerative colitis (UC). We evaluated the efficacy and safety of mirikizumab in a Japanese subpopulation with moderately to severely active UC from the LUCENT-1 and LUCENT-2 studies.
Methods
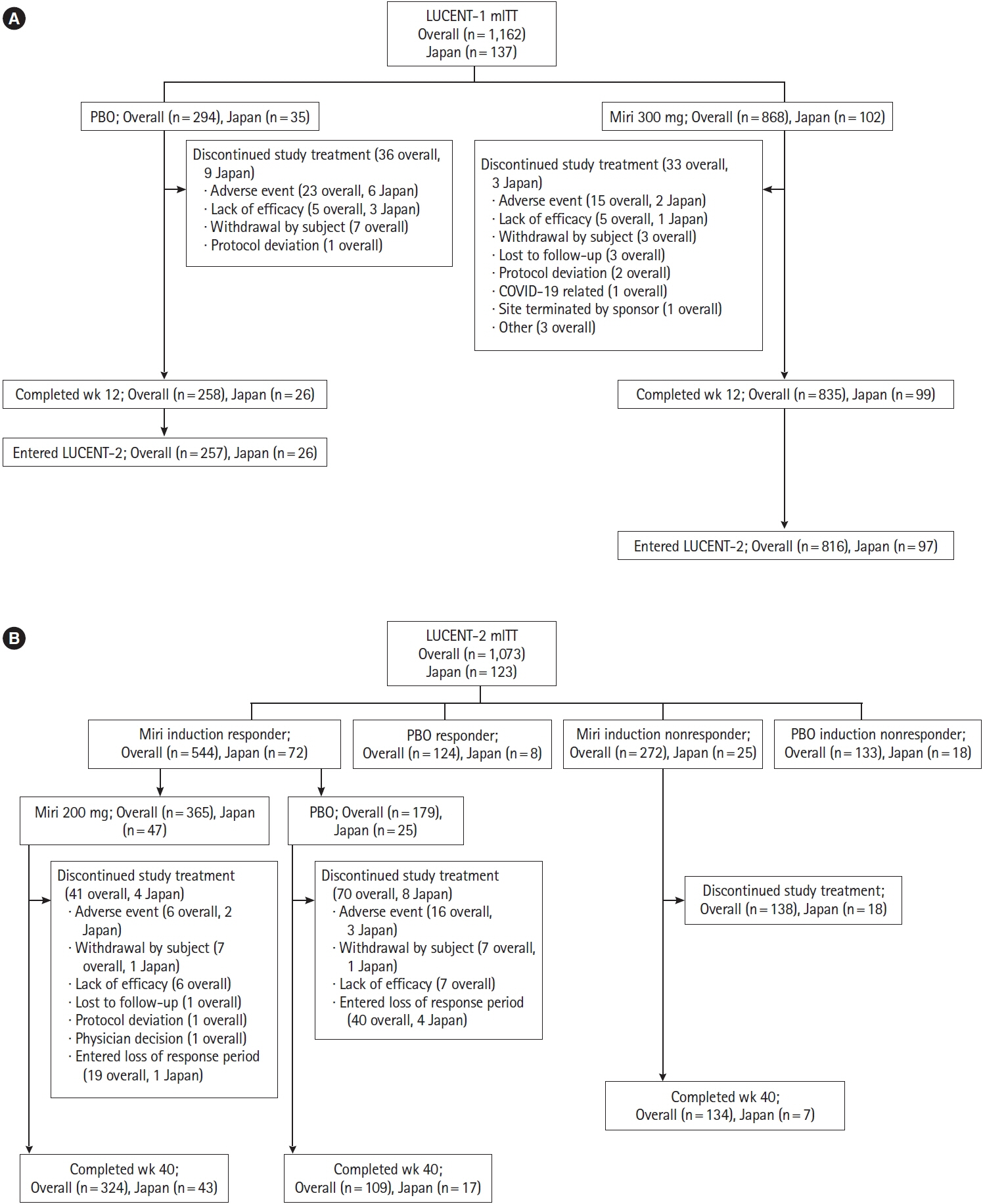
LUCENT-1 and LUCENT-2 were phase 3, randomized, double-blind, placebo-controlled trials of mirikizumab therapy in adults with moderately to severely active UC. LUCENT-1 was a 12-week induction trial where patients were randomized 3:1 to receive intravenous mirikizumab 300 mg or placebo every 4 weeks (Q4W). Patients achieving a clinical response with mirikizumab following the induction study were re-randomized 2:1 to double-blind treatment with either mirikizumab 200 mg or placebo subcutaneously Q4W during the 40-week maintenance study. The primary outcomes were clinical remission at week 12 of LUCENT-1 and week 40 of LUCENT-2.
Results
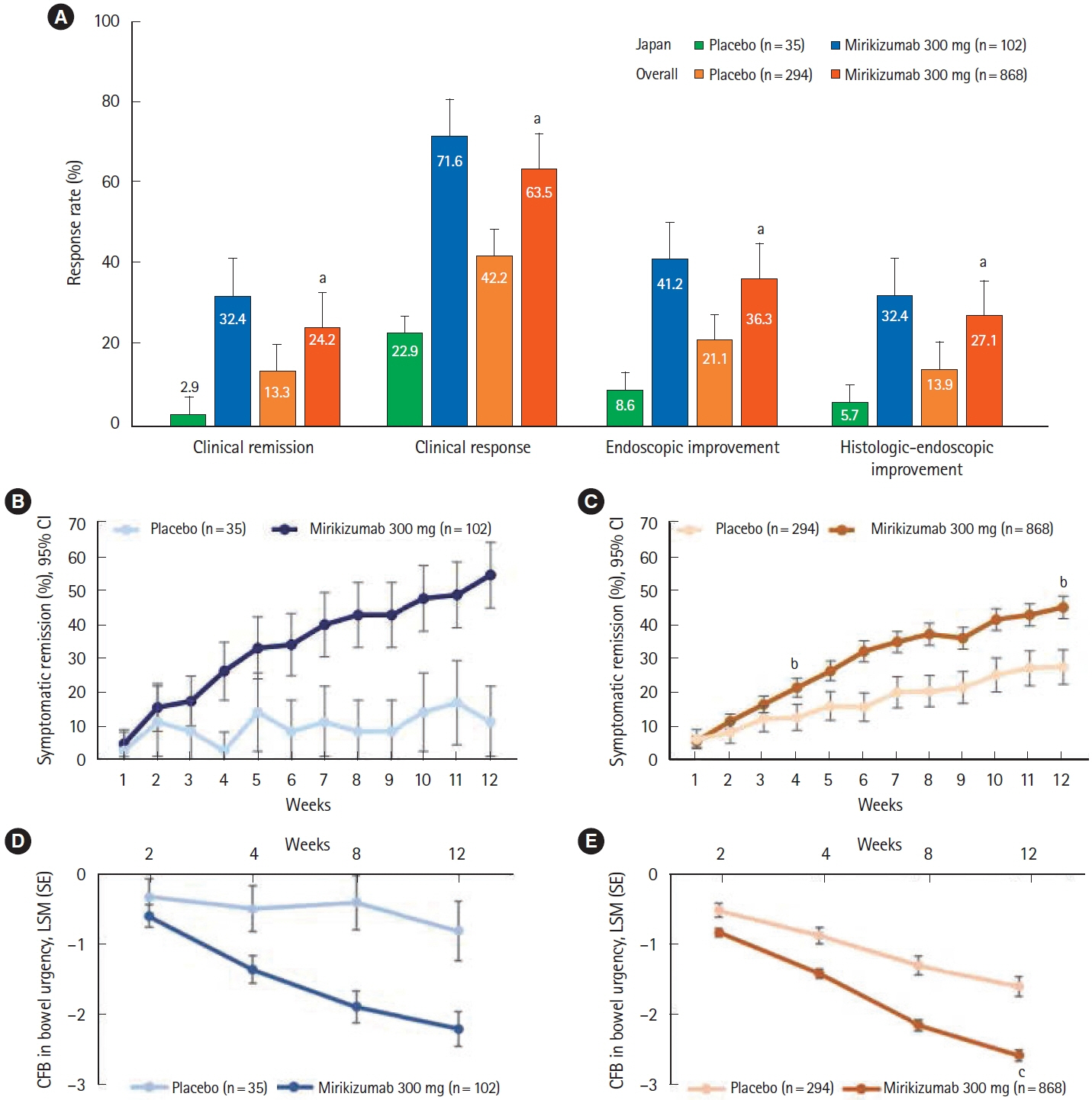
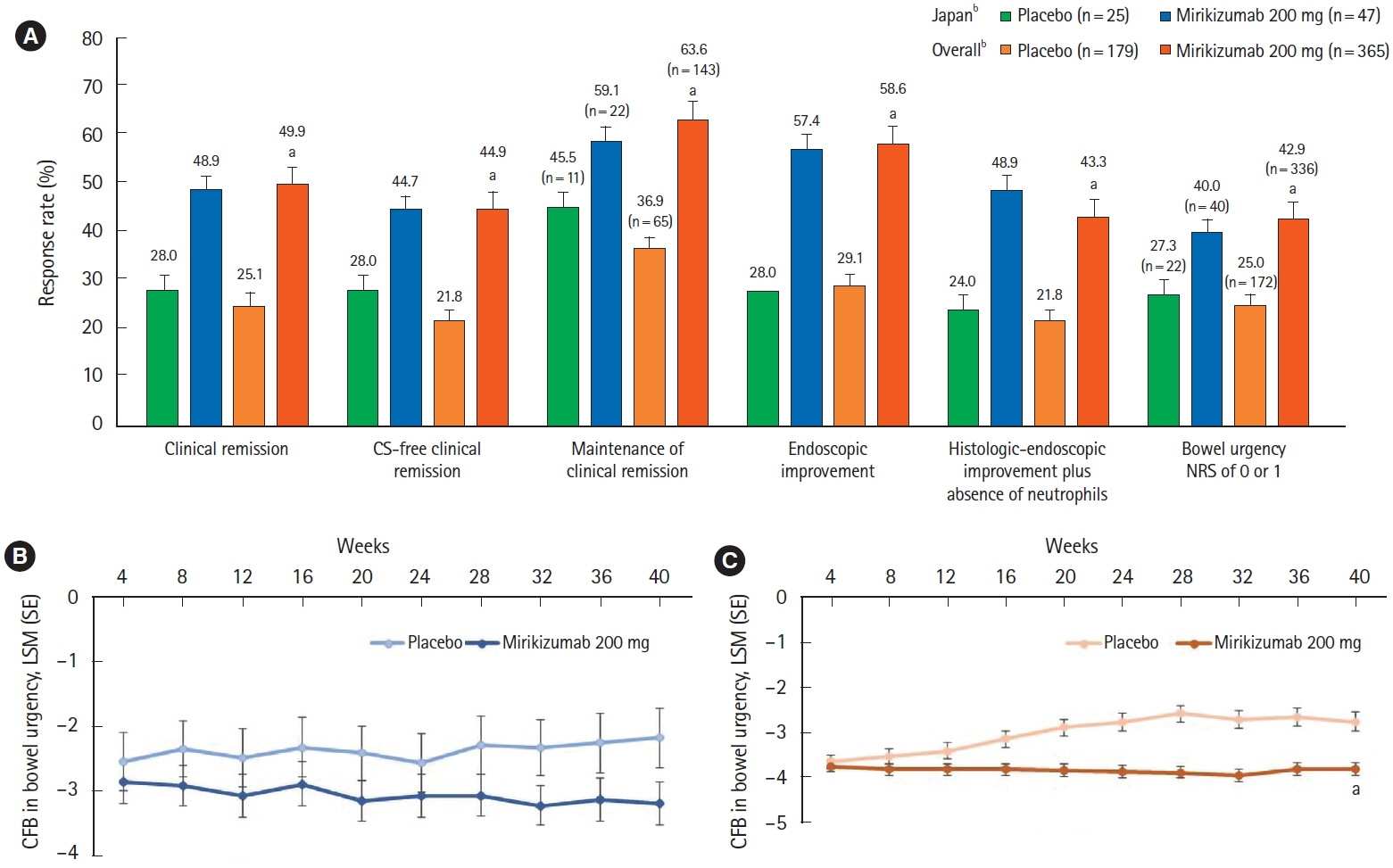
A total of 137 patients enrolled in Japan were randomized to mirikizumab (n = 102) or placebo (n = 35). Compared with placebo, patients who received mirikizumab showed numerically higher clinical remission at week 12 of induction (32.4% [n = 33] vs. 2.9% [n = 1]) and at week 40 of maintenance (48.9% [n = 23] vs. 28.0% [n = 7]). A greater number of patients achieved key secondary endpoints in the mirikizumab group compared with placebo. The frequency of treatment-emergent adverse events was similar across mirikizumab and placebo groups. Efficacy and safety results observed in the Japanese subpopulation were generally consistent with those in the overall population.
Conclusions
Mirikizumab induction and maintenance treatments were effective in Japanese patients with moderately to severely active UC. No new safety concerns were identified.
Figure
Reference
-
1. Kobayashi T, Siegmund B, Le Berre C, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020; 6:74.
Article2. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017; 389:1756–1770.
Article3. Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: a narrative review. Dig Liver Dis. 2021; 53:803–808.
Article4. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017; 390:2769–2778.
Article5. Murakami Y, Nishiwaki Y, Oba MS, et al. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: an analysis of a nationwide survey. J Gastroenterol. 2019; 54:1070–1077.
Article6. Gordon JP, McEwan PC, Maguire A, Sugrue DM, Puelles J. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol. 2015; 27:804–812.
Article7. Kobayashi T, Okamoto S, Hisamatsu T, et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008; 57:1682–1689.
Article8. Oppmann B, Lesley R, Blom B, et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000; 13:715–725.
Article9. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022; 20:105–115.
Article10. Sandborn WJ, Ferrante M, Bhandari BR, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020; 158:537–549.
Article11. D’Haens G, Dubinsky M, Kobayashi T, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2023; 388:2444–2455.
Article12. Hibi T, Ishibashi T, Ikenoue Y, Yoshihara R, Nihei A, Kobayashi T. Ulcerative colitis: disease burden, impact on daily life, and reluctance to consult medical professionals: results from a Japanese Internet Survey. Inflamm Intest Dis. 2020; 5:27–35.
Article13. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021; 160:1570–1583.
Article14. Jangi S, Yoon H, Dulai PS, et al. Predictors and outcomes of histological remission in ulcerative colitis treated to endoscopic healing. Aliment Pharmacol Ther. 2020; 52:1008–1016.
Article15. Pai RK, Hartman DJ, Rivers CR, et al. Complete resolution of mucosal neutrophils associates with improved long-term clinical outcomes of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2020; 18:2510–2517.
Article16. Liu JZ, van Sommeren S, Huang H, et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015; 47:979–986.
Article17. Sands BE, Peyrin-Biroulet L, Kierkus J, et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022; 162:495–508.
Article18. Blauvelt A, Kimball AB, Augustin M, et al. Efficacy and safety of mirikizumab in psoriasis: results from a 52-week, doubleblind, placebo-controlled, randomized withdrawal, phase III trial (OASIS-1). Br J Dermatol. 2022; 187:866–877.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and safety of filgotinib as induction and maintenance therapy for Japanese patients with moderately to severely active ulcerative colitis: a post-hoc analysis of the phase 2b/3 SELECTION trial
- Efficacy and safety of ustekinumab in East Asian patients with moderately to severely active ulcerative colitis: a subpopulation analysis of global phase 3 induction and maintenance studies (UNIFI)
- Efficacy of biologic therapies for biologic-naïve Japanese patients with moderately to severely active ulcerative colitis: a network meta-analysis
- Efficacy and Safety of Adalimumab in Moderately to Severely Active Cases of Ulcerative Colitis: A Meta-Analysis of Published Placebo-Controlled Trials
- Efficacy and safety of ustekinumab in Japanese patients with moderately to severely active Crohn's disease: a subpopulation analysis of phase 3 induction and maintenance studies