J Rheum Dis.
2024 Apr;31(2):97-107. 10.4078/jrd.2023.0056.
Machine learning models with time-series clinical features to predict radiographic progression in patients with ankylosing spondylitis
- Affiliations
-
- 1Department of Internal Medicine, Inje University Ilsan Paik Hospital, Inje University College of Medicine, Seoul, Korea
- 2Department of Biomedical Engineering, Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Information Medicine, Big Data Research Center, Asan Medical Center, Seoul, Korea
- 5Department of Radiology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- 6Departments of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 7Departments of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 8Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea
- KMID: 2554325
- DOI: http://doi.org/10.4078/jrd.2023.0056
Abstract
Objective
Ankylosing spondylitis (AS) is chronic inflammatory arthritis causing structural damage and radiographic progression to the spine due to repeated and continuous inflammation over a long period. This study establishes the application of machine learning models to predict radiographic progression in AS patients using time-series data from electronic medical records (EMRs).
Methods
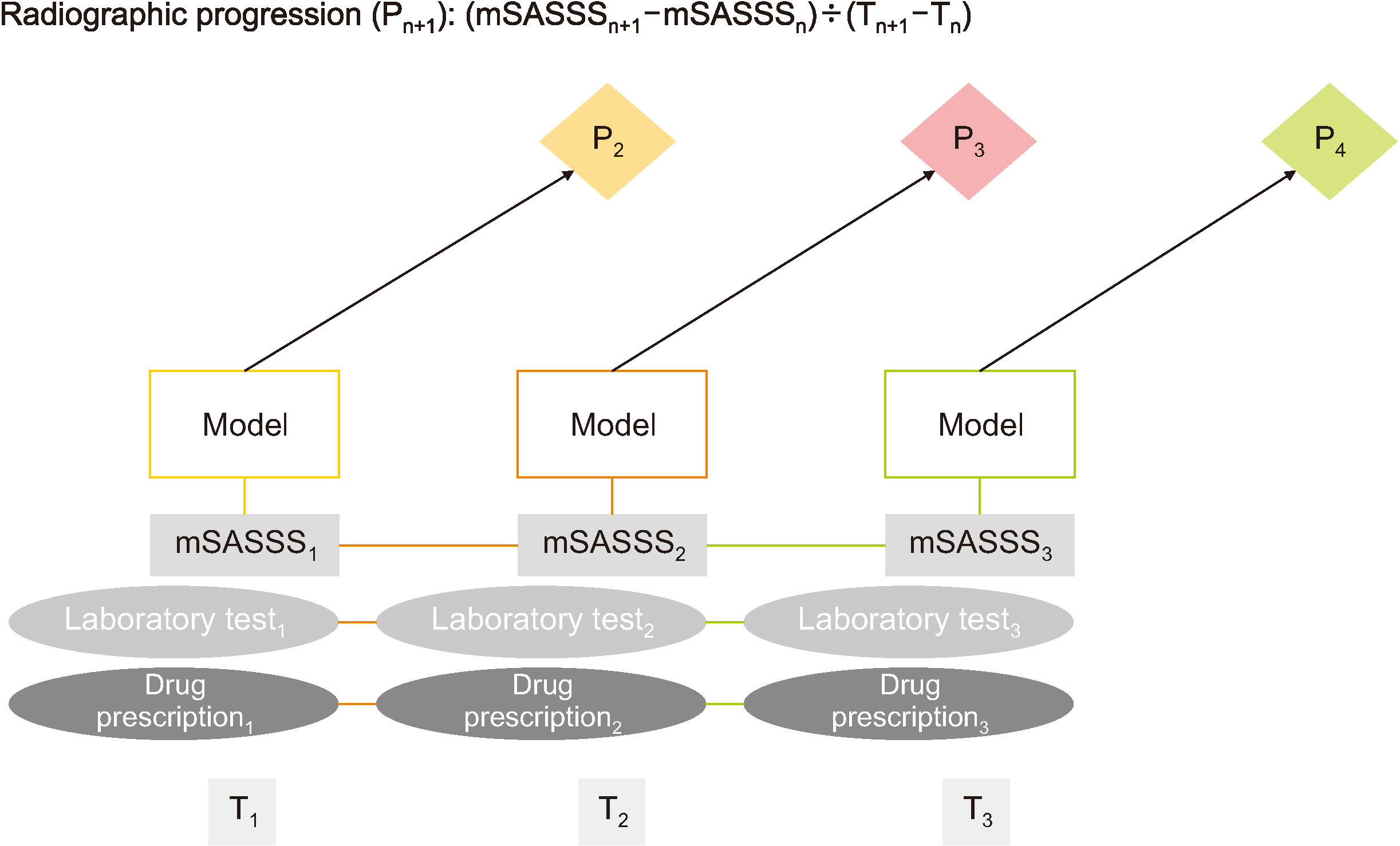
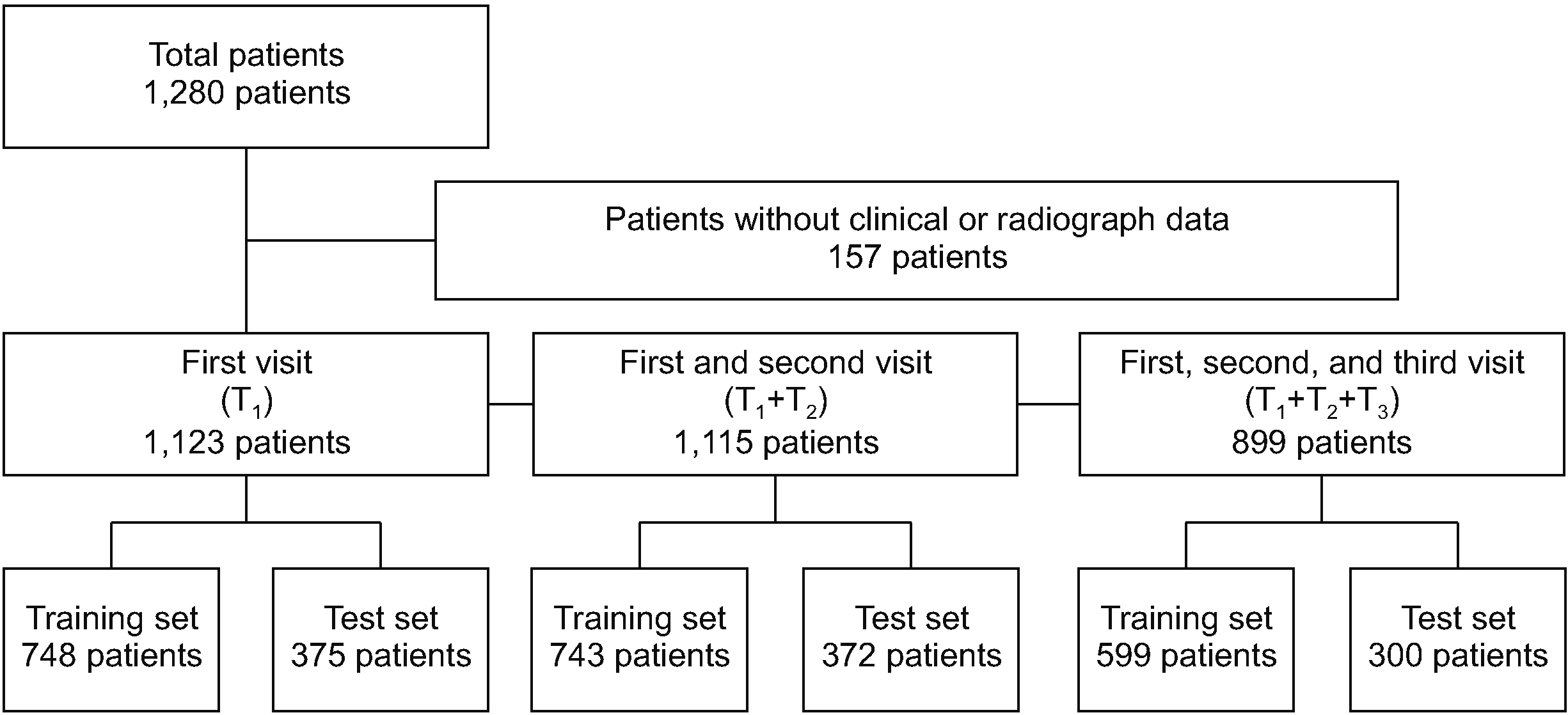
EMR data, including baseline characteristics, laboratory findings, drug administration, and modified Stoke AS Spine Score (mSASSS), were collected from 1,123 AS patients between January 2001 and December 2018 at a single center at the time of first (T1 ), second (T2 ), and third (T3 ) visits. The radiographic progression of the (n+1)th visit (Pn+1 =(mSASSSn+1 –mSASSSn )/(Tn+1 – Tn )≥1 unit per year) was predicted using follow-up visit datasets from T1 to Tn . We used three machine learning methods (logistic regression with the least absolute shrinkage and selection operation, random forest, and extreme gradient boosting algorithms) with three-fold cross-validation.
Results
The random forest model using the T1 EMR dataset best predicted the radiographic progression P2 among the machine learning models tested with a mean accuracy and area under the curves of 73.73% and 0.79, respectively. Among the T1 variables, the most important variables for predicting radiographic progression were in the order of total mSASSS, age, and alkaline phosphatase.
Conclusion
Prognosis predictive models using time-series data showed reasonable performance with clinical features of the first visit dataset when predicting radiographic progression.
Figure
Reference
-
1. Inman RD. 2021; Axial spondyloarthritis: current advances, future challenges. J Rheum Dis. 28:55–9. DOI: 10.4078/jrd.2021.28.2.55. PMID: 37476012. PMCID: PMC10324891.
Article2. Brown MA, Li Z, Cao KL. 2020; Biomarker development for axial spondyloarthritis. Nat Rev Rheumatol. 16:448–63. DOI: 10.1038/s41584-020-0450-0. PMID: 32606474.
Article3. Lorenzin M, Ometto F, Ortolan A, Felicetti M, Favero M, Doria A, et al. 2020; An update on serum biomarkers to assess axial spondyloarthritis and to guide treatment decision. Ther Adv Musculoskelet Dis. 12:1759720X20934277. DOI: 10.1177/1759720X20934277. PMID: 32636944. PMCID: PMC7315656.
Article4. Rademacher J, Tietz LM, Le L, Hartl A, Hermann KA, Sieper J, et al. 2019; Added value of biomarkers compared with clinical parameters for the prediction of radiographic spinal progression in axial spondyloarthritis. Rheumatology (Oxford). 58:1556–64. DOI: 10.1093/rheumatology/kez025. PMID: 30830164.
Article5. Beam AL, Kohane IS. 2018; Big data and machine learning in health care. JAMA. 319:1317–8. DOI: 10.1001/jama.2017.18391. PMID: 29532063.
Article6. Fontanella S, Cucco A, Custovic A. 2021; Machine learning in asthma research: moving toward a more integrated approach. Expert Rev Respir Med. 15:609–21. DOI: 10.1080/17476348.2021.1894133. PMID: 33618597.
Article7. Beckmann JS, Lew D. 2016; Reconciling evidence-based medicine and precision medicine in the era of big data: challenges and opportunities. Genome Med. 8:134. DOI: 10.1186/s13073-016-0388-7. PMID: 27993174. PMCID: PMC5165712.
Article8. Lee CH, Yoon HJ. 2017; Medical big data: promise and challenges. Kidney Res Clin Pract. 36:3–11. DOI: 10.23876/j.krcp.2017.36.1.3. PMID: 28392994. PMCID: PMC5331970.
Article9. Rumsfeld JS, Joynt KE, Maddox TM. 2016; Big data analytics to improve cardiovascular care: promise and challenges. Nat Rev Cardiol. 13:350–9. DOI: 10.1038/nrcardio.2016.42. PMID: 27009423.
Article10. van der Linden S, Valkenburg HA, Cats A. 1984; Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27:361–8. DOI: 10.1002/art.1780270401. PMID: 6231933.11. Creemers MC, Franssen MJ, Gribnau FW, van de Putte LB, van Riel PL. van't Hof MA. 2005; Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis. 64:127–9. DOI: 10.1136/ard.2004.020503. PMID: 15051621. PMCID: PMC1755183.
Article12. van der Heijde D, Braun J, Deodhar A, Baraliakos X, Landewé R, Richards HB, et al. 2019; Modified stoke ankylosing spondylitis spinal score as an outcome measure to assess the impact of treatment on structural progression in ankylosing spondylitis. Rheumatology (Oxford). 58:388–400. DOI: 10.1093/rheumatology/key128. PMID: 29860356. PMCID: PMC6381766.
Article13. Koo BS, Oh JS, Park SY, Shin JH, Ahn GY, Lee S, et al. 2020; Tumour necrosis factor inhibitors slow radiographic progression in patients with ankylosing spondylitis: 18-year real-world evidence. Ann Rheum Dis. 79:1327–32. DOI: 10.1136/annrheumdis-2019-216741. PMID: 32660979.
Article14. Lee TH, Koo BS, Nam B, Oh JS, Park SY, Lee S, et al. 2020; Conventional disease-modifying antirheumatic drugs therapy may not slow spinal radiographic progression in ankylosing spondylitis: results from an 18-year longitudinal dataset. Ther Adv Musculoskelet Dis. 12:1759720X20975912. DOI: 10.1177/1759720X20975912. PMID: 33294039. PMCID: PMC7705797.
Article15. Haroon N, Inman RD, Learch TJ, Weisman MH, Lee M, Rahbar MH, et al. 2013; The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 65:2645–54. DOI: 10.1002/art.38070. PMID: 23818109. PMCID: PMC3974160.
Article16. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. 2011; Scikit-learn: machine learning in Python. J Mach Learn Res. 12:2825–30.17. Breiman L. 2001; Random forests. Mach Learn. 45:5–32. DOI: 10.1023/A:1010933404324.18. Sheridan RP, Wang WM, Liaw A, Ma J, Gifford EM. 2016; Extreme gradient boosting as a method for quantitative structure-activity relationships. J Chem Inf Model. 56:2353–60. Erratum in: J Chem Inf Model 2020;60:1910. DOI: 10.1021/acs.jcim.6b00591. PMID: 27958738.
Article19. Kim JH. 2009; Estimating classification error rate: repeated cross-validation, repeated hold-out and bootstrap. Comput Stat Data Anal. 53:3735–45. DOI: 10.1016/j.csda.2009.04.009.
Article20. Greenwell BM, Boehmke BC, McCarthy AJ. 2018. A simple and effective model-based variable importance measure. ArXiv [Preprint]. https://doi.org/10.48550/arXiv.1805.04755. cited 2021 Jun 15.
Article21. Kuhn M, Johnson K. 2013. Applied predictive modeling. Springer;New York: DOI: 10.1007/978-1-4614-6849-3.22. Poddubnyy D, Haibel H, Listing J, Märker-Hermann E, Zeidler H, Braun J, et al. 2012; Baseline radiographic damage, elevated acute-phase reactant levels, and cigarette smoking status predict spinal radiographic progression in early axial spondylarthritis. Arthritis Rheum. 64:1388–98. DOI: 10.1002/art.33465. PMID: 22127957.
Article23. Poddubnyy D, Protopopov M, Haibel H, Braun J, Rudwaleit M, Sieper J. 2016; High disease activity according to the Ankylosing Spondylitis Disease Activity Score is associated with accelerated radiographic spinal progression in patients with early axial spondyloarthritis: results from the GErman SPondyloarthritis Inception Cohort. Ann Rheum Dis. 75:2114–8. DOI: 10.1136/annrheumdis-2016-209209. PMID: 27125522.
Article24. Poddubnyy DA, Rudwaleit M, Listing J, Braun J, Sieper J. 2010; Comparison of a high sensitivity and standard C reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis. 69:1338–41. DOI: 10.1136/ard.2009.120139. PMID: 20498207.
Article25. Ramiro S, van der Heijde D, van Tubergen A, Stolwijk C, Dougados M, van den Bosch F, et al. 2014; Higher disease activity leads to more structural damage in the spine in ankylosing spondylitis: 12-year longitudinal data from the OASIS cohort. Ann Rheum Dis. 73:1455–61. DOI: 10.1136/annrheumdis-2014-205178. PMID: 24812292.
Article26. Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P. 2017; Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nat Rev Nephrol. 13:429–42. DOI: 10.1038/nrneph.2017.60. PMID: 28502983.
Article27. Cheung BM, Ong KL, Cheung RV, Wong LY, Wat NM, Tam S, et al. 2008; Association between plasma alkaline phosphatase and C-reactive protein in Hong Kong Chinese. Clin Chem Lab Med. 46:523–7. DOI: 10.1515/CCLM.2008.111. PMID: 18605934.
Article28. Damera S, Raphael KL, Baird BC, Cheung AK, Greene T, Beddhu S. 2011; Serum alkaline phosphatase levels associate with elevated serum C-reactive protein in chronic kidney disease. Kidney Int. 79:228–33. DOI: 10.1038/ki.2010.356. PMID: 20881941. PMCID: PMC5260661.
Article29. Kang KY, Hong YS, Park SH, Ju JH. 2015; Increased serum alkaline phosphatase levels correlate with high disease activity and low bone mineral density in patients with axial spondyloarthritis. Semin Arthritis Rheum. 45:202–7. DOI: 10.1016/j.semarthrit.2015.03.002. PMID: 25895696.
Article30. Che Z, Purushotham S, Cho K, Sontag D, Liu Y. 2018; Recurrent neural networks for multivariate time series with missing values. Sci Rep. 8:6085. DOI: 10.1038/s41598-018-24271-9. PMID: 29666385. PMCID: PMC5904216.
Article31. Koo BS, Lee S, Oh JS, Park SY, Ahn GY, Shin JH, et al. 2022; Early control of C-reactive protein levels with non-biologics is associated with slow radiographic progression in radiographic axial spondyloarthritis. Int J Rheum Dis. 25:311–6. DOI: 10.1111/1756-185X.14268. PMID: 34935282.
Article32. Walsh JA, Rozycki M, Yi E, Park Y. 2019; Application of machine learning in the diagnosis of axial spondyloarthritis. Curr Opin Rheumatol. 31:362–7. DOI: 10.1097/BOR.0000000000000612. PMID: 31033569. PMCID: PMC6553337.
Article33. Deodhar A, Rozycki M, Garges C, Shukla O, Arndt T, Grabowsky T, et al. 2020; Use of machine learning techniques in the development and refinement of a predictive model for early diagnosis of ankylosing spondylitis. Clin Rheumatol. 39:975–82. DOI: 10.1007/s10067-019-04553-x. PMID: 31044386.
Article34. Walsh JA, Pei S, Penmetsa G, Hansen JL, Cannon GW, Clegg DO, et al. 2020; Identification of axial spondyloarthritis patients in a large dataset: the development and validation of novel methods. J Rheumatol. 47:42–9. DOI: 10.3899/jrheum.181005. PMID: 30877217.
Article35. Walsh JA, Pei S, Penmetsa GK, Leng J, Cannon GW, Clegg DO, et al. 2018; Cohort identification of axial spondyloarthritis in a large healthcare dataset: current and future methods. BMC Musculoskelet Disord. 19:317. DOI: 10.1186/s12891-018-2211-7. PMID: 30185185. PMCID: PMC6123987.
Article36. Walsh JA, Shao Y, Leng J, He T, Teng CC, Redd D, et al. 2017; Identifying axial spondyloarthritis in electronic medical records of US veterans. Arthritis Care Res (Hoboken). 69:1414–20. DOI: 10.1002/acr.23140. PMID: 27813310.
Article37. Bressem KK, Vahldiek JL, Adams L, Niehues SM, Haibel H, Rodriguez VR, et al. 2021; Deep learning for detection of radiographic sacroiliitis: achieving expert-level performance. Arthritis Res Ther. 23:106. DOI: 10.1186/s13075-021-02484-0. PMID: 33832519. PMCID: PMC8028815.
Article38. Joo YB, Baek IW, Park YJ, Park KS, Kim KJ. 2020; Machine learning-based prediction of radiographic progression in patients with axial spondyloarthritis. Clin Rheumatol. 39:983–91. DOI: 10.1007/s10067-019-04803-y. PMID: 31667645.
Article39. Rajkomar A, Dean J, Kohane I. 2019; Machine learning in medicine. N Engl J Med. 380:1347–58. DOI: 10.1056/NEJMra1814259. PMID: 30943338.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinieal Values of Single Photon Emission Computed Tomography ( SPECT ) in Ankylosing Spondylitis
- Treatment of Cervical Cord Injury with Ankylosing Spondylitis: Case Report
- Clinical features of ankylosing spondylitis
- Ankylosing Spondylitis: Prevention And Surgical Correction Of Deformity
- Predictive factors of radiographic progression in ankylosing spondylitis