J Rheum Dis.
2024 Apr;31(2):68-78. 10.4078/jrd.2023.0084.
Medical treatment of osteoarthritis: botanical pharmacologic aspect
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Dong-A University Hospital, Busan, Korea
- KMID: 2554322
- DOI: http://doi.org/10.4078/jrd.2023.0084
Abstract
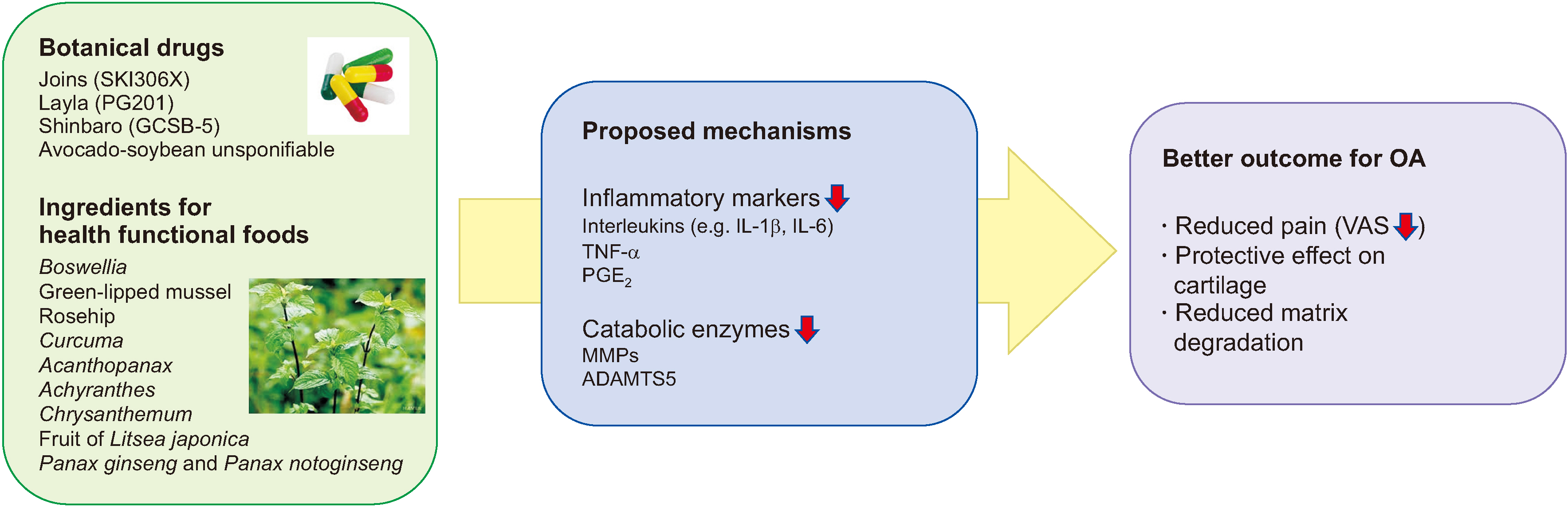
- Osteoarthritis (OA) is the most common form of arthritis, and its prevalence is expected to further increase as our society ages. Despite many approaches to cure OA, no drugs are currently proven to modulate the progression of OA. Nowadays, new OA treatment options are holistically developed and one of the approaches of treatment option is botanical drugs. Some botanical drugs for OA have shown both therapeutic effect comparable to refined drugs in small studies and fewer side effects. Hence, there are various health functional foods which are known to relieve symptoms of OA. However, since there are many botanical products, clinicians are not familiar to the efficacy of each botanical product, making it challenging to use them appropriately in clinical practice. Here, we summarize the botanical products available for treating OA, including prescription botanical drugs and health functional foods available in Korea. Further studies and the purification of effective molecules from botanical products will be necessary in future.
Keyword
Figure
Reference
-
1. Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, et al. 2019; OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 27:1578–89. DOI: 10.1016/j.joca.2019.06.011. PMID: 31278997.
Article2. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2020; 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72:220–33. Erratum in: Arthritis Rheumatol 2021;73:799. DOI: 10.1002/art.41142. PMID: 31908163. PMCID: PMC10518852.
Article3. Kloppenburg M, Kroon FP, Blanco FJ, Doherty M, Dziedzic KS, Greibrokk E, et al. 2019; 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis. 78:16–24. DOI: 10.1136/annrheumdis-2018-213826. PMID: 30154087.
Article4. Choi JH, Choi JH, Kim DY, Yoon JH, Youn HY, Yi JB, et al. 2002; Effects of SKI 306X, a new herbal agent, on proteoglycan degradation in cartilage explant culture and collagenase-induced rabbit osteoarthritis model. Osteoarthritis Cartilage. 10:471–8. DOI: 10.1053/joca.2002.0526. PMID: 12056850.
Article5. Lung YB, Seong SC, Lee MC, Shin YU, Kim DH, Kim JM, et al. 2004; A four-week, randomized, double-blind trial of the efficacy and safety of SKI306X: a herbal anti-arthritic agent versus diclofenac in osteoarthritis of the knee. Am J Chin Med. 32:291–301. DOI: 10.1142/S0192415X04001941. PMID: 15315266.
Article6. Hartog A, Hougee S, Faber J, Sanders A, Zuurman C, Smit HF, et al. 2008; The multicomponent phytopharmaceutical SKI306X inhibits in vitro cartilage degradation and the production of inflammatory mediators. Phytomedicine. 15:313–20. DOI: 10.1016/j.phymed.2007.09.005. PMID: 17949960.
Article7. Choi CH, Kim TH, Sung YK, Choi CB, Na YI, Yoo H, et al. 2014; SKI306X inhibition of glycosaminoglycan degradation in human cartilage involves down-regulation of cytokine-induced catabolic genes. Korean J Intern Med. 29:647–55. DOI: 10.3904/kjim.2014.29.5.647. PMID: 25228841. PMCID: PMC4164729.
Article8. Woo Y, Hyun MK. 2017; Evaluation of cardiovascular risk associated with SKI306X use in patients with osteoarthritis and rheumatoid arthritis. J Ethnopharmacol. 207:42–6. DOI: 10.1016/j.jep.2017.06.003. PMID: 28602866.
Article9. Kim HR, Kim KW, Kim BM, Won JY, Min HK, Lee KA, et al. 2019; Regulation of Th17 cytokine-induced osteoclastogenesis via SKI306X in rheumatoid arthritis. J Clin Med. 8:1012. DOI: 10.3390/jcm8071012. PMID: 31295961. PMCID: PMC6678573.
Article10. Choi J, Kim SH, Kim S. 2012; Suppressive effects of PG201, an antiarthritic botanical formulation, on lipopolysaccharide-induced inflammatory mediators in Raw264.7 cells. Exp Biol Med (Maywood). 237:499–508. DOI: 10.1258/ebm.2011.011203. PMID: 22442340.
Article11. Shin SS, Jin M, Jung HJ, Kim B, Jeon H, Choi JJ, et al. 2003; Suppressive effects of PG201, an ethanol extract from herbs, on collagen-induced arthritis in mice. Rheumatology (Oxford). 42:665–72. DOI: 10.1093/rheumatology/keg209. PMID: 12709543.
Article12. Park KC, Park EJ, Kim ER, Kim Y, Chung SH, Cho BW, et al. 2005; Therapeutic effects of PG201, an ethanol extract from herbs, through cartilage protection on collagenase-induced arthritis in rabbits. Biochem Biophys Res Commun. 331:1469–77. DOI: 10.1016/j.bbrc.2005.04.030. PMID: 15883039.
Article13. Yoo WH, Yoo HG, Park SH, Baek HJ, Lee YJ, Shim SC, et al. 2014; Efficacy and safety of PG201 (Layla(®)) and celecoxib in the treatment of symptomatic knee osteoarthritis: a double-blinded, randomized, multi-center, active drug comparative, parallel-group, non-inferiority, phase III study. Rheumatol Int. 34:1369–78. DOI: 10.1007/s00296-014-2964-8. PMID: 24531687.
Article14. Ha CW, Park YB, Min BW, Han SB, Lee JH, Won YY, et al. 2016; Prospective, randomized, double-blinded, double-dummy and multicenter phase IV clinical study comparing the efficacy and safety of PG201 (Layla) and SKI306X in patients with osteoarthritis. J Ethnopharmacol. 181:1–7. DOI: 10.1016/j.jep.2016.01.029. PMID: 26821189.
Article15. Choi J, Lee J, Lee J, Kim SH, Kim J, Kim S. 2013; PG201 downregulates the production of nitrite by upregulating heme oxygenase-1 expression through the control of phosphatidylinositol 3-kinase and NF-E2-related factor 2. Nitric Oxide. 33:42–55. DOI: 10.1016/j.niox.2013.05.003. PMID: 23747519.
Article16. Kim HJ, Kim HM, Ryu B, Lee WS, Shin JS, Lee KT, et al. 2016; Constituents of PG201 (Layla(®)), a multi-component phytopharmaceutical, with inhibitory activity on LPS-induced nitric oxide and prostaglandin E2 productions in macrophages. Arch Pharm Res. 39:231–9. DOI: 10.1007/s12272-015-0654-z. PMID: 26306655.
Article17. Bae MJ, Choi J, Kim HK, Lim S, Kim S. 2018; PG201 protects mice from experimental autoimmune encephalomyelitis via suppression of effector T cell activation. Phytomedicine. 43:150–7. DOI: 10.1016/j.phymed.2018.04.026. PMID: 29747748.
Article18. Kim JK, Park SW, Kang JW, Kim YJ, Lee SY, Shin J, et al. 2012; Effect of GCSB-5, a herbal formulation, on monosodium iodoacetate-induced osteoarthritis in rats. Evid Based Complement Alternat Med. 2012:730907. DOI: 10.1155/2012/730907. PMID: 22474519. PMCID: PMC3303749.
Article19. Wang CC, Chen LG, Yang LL. 1999; Inducible nitric oxide synthase inhibitor of the Chinese herb I. Saposhnikovia divaricata (Turcz.) Schischk. Cancer Lett. 145:151–7. DOI: 10.1016/S0304-3835(99)00248-7. PMID: 10530783.
Article20. Makarov SS. 2001; NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 3:200–6. DOI: 10.1186/ar300. PMID: 11438035. PMCID: PMC128895.21. Zhu J, Gao X, Xie WL, Jin YZ, Sun WJ. 2005; [Effect of geniposide on serum IL-1beta and TNF-alpha of rheumatoid arthritis rats]. Zhongguo Zhong Yao Za Zhi. 30:708–11. Chinese.22. Kim BH, Park KS, Chang IM. 2009; Elucidation of anti-inflammatory potencies of Eucommia ulmoides bark and Plantago asiatica seeds. J Med Food. 12:764–9. DOI: 10.1089/jmf.2008.1239. PMID: 19735174.23. Chung HJ, Lee HS, Shin JS, Lee SH, Park BM, Youn YS, et al. 2010; Modulation of acute and chronic inflammatory processes by a traditional medicine preparation GCSB-5 both in vitro and in vivo animal models. J Ethnopharmacol. 130:450–9. DOI: 10.1016/j.jep.2010.05.020. PMID: 20621661.
Article24. Lee CH, Kim SH, Lee JS, Cho KH, Kim JS, Cho SH, et al. 2005; Evaluation of the antinociceptive properties of GCSB-5, a herbal formulation. Korean J Pharmacogn. 36:299–304.25. Park YG, Ha CW, Han CD, Bin SI, Kim HC, Jung YB, et al. 2013; A prospective, randomized, double-blind, multicenter comparative study on the safety and efficacy of Celecoxib and GCSB-5, dried extracts of six herbs, for the treatment of osteoarthritis of knee joint. J Ethnopharmacol. 149:816–24. DOI: 10.1016/j.jep.2013.08.008. PMID: 23954277.
Article26. Ha CW, Park YB, Kyung HS, Han CS, Bae KC, Lim HC, et al. 2016; Gastrointestinal safety and efficacy of long-term GCSB-5 use in patients with osteoarthritis: a 24-week, multicenter study. J Ethnopharmacol. 189:310–8. DOI: 10.1016/j.jep.2016.05.031. PMID: 27196293.
Article27. Park JK, Shin K, Kang EH, Ha YJ, Lee YJ, Lee KH, et al. 2016; Efficacy and tolerability of GCSB-5 for hand osteoarthritis: a randomized, controlled trial. Clin Ther. 38:1858–68.e2. DOI: 10.1016/j.clinthera.2016.06.016. PMID: 27449412.
Article28. Cho HK, Kim SY, Choi MJ, Baek SO, Kwak SG, Ahn SH. 2016; The effect of GCSB-5 a new herbal medicine on changes in pain behavior and neuroglial activation in a rat model of lumbar disc herniation. J Korean Neurosurg Soc. 59:98–105. DOI: 10.3340/jkns.2016.59.2.98. PMID: 26962414. PMCID: PMC4783491.
Article29. Kim WK, Shin JS, Lee J, Koh W, Ha IH, Park HJ, et al. 2023; Effects of the administration of Shinbaro 2 in a rat lumbar disk herniation model. Front Neurol. 14:4104472. DOI: 10.3389/fneur.2023.1044724. PMID: 36970511. PMCID: PMC10036394.
Article30. Bang J, Kim G, Park SY, Jung HR, Kim SH, Kim JM. 2023; GCSB-5 regulates inflammatory arthritis and pain by modulating the mitogen-activated protein kinase signaling pathway in a murine model of rheumatoid arthritis. Arch Rheumatol. 38:566–78. DOI: 10.46497/ArchRheumatol.2023.9643. PMID: 38125068. PMCID: PMC10728744.
Article31. Lippiello L, Nardo JV, Harlan R, Chiou T. 2008; Metabolic effects of avocado/soy unsaponifiables on articular chondrocytes. Evid Based Complement Alternat Med. 5:191–7. DOI: 10.1093/ecam/nem132. PMID: 18604259. PMCID: PMC2396479.
Article32. Henrotin YE, Labasse AH, Jaspar JM, De Groote DD, Zheng SX, Guillou GB, et al. 1998; Effects of three avocado/soybean unsaponifiable mixtures on metalloproteinases, cytokines and prostaglandin E2 production by human articular chondrocytes. Clin Rheumatol. 17:31–9. DOI: 10.1007/BF01450955. PMID: 9586676.
Article33. Kharazmi A. 2008; Laboratory and preclinical studies on the anti-inflammatory and anti-oxidant properties of rosehip powder - identification and characterization of the active component GOPO®. Osteoarthritis Cartilage. 16(Suppl 1):S5–7. DOI: 10.1016/S1063-4584(08)60003-5.
Article34. Simental-Mendía M, Sánchez-García A, Acosta-Olivo CA, Vilchez-Cavazos F, Osuna-Garate J, Peña-Martínez VM, et al. 2019; Efficacy and safety of avocado-soybean unsaponifiables for the treatment of hip and knee osteoarthritis: a systematic review and meta-analysis of randomized placebo-controlled trials. Int J Rheum Dis. 22:1607–15. DOI: 10.1111/1756-185X.13658. PMID: 31328413.
Article35. Basch E, Boon H, Davies-Heerema T, Foppo I, Hashmi S, Hasskarl J, et al. 2004; Boswellia: an evidence-based systematic review by the Natural Standard Research Collaboration. J Herb Pharmacother. 4:63–83. DOI: 10.1080/J157v04n03_06.36. Badria FA, El-Farahaty T, Shabana AA, Hawas SA, El-Batoty MF. 2002; Boswellia-curcumin preparation for treating knee osteoarthritis: a clinical evaluation. Altern Complement Ther. 8:341–8. DOI: 10.1089/107628002761574635.
Article37. Bannuru RR, Osani MC, Al-Eid F, Wang C. 2018; Efficacy of curcumin and Boswellia for knee osteoarthritis: systematic review and meta-analysis. Semin Arthritis Rheum. 48:416–29. DOI: 10.1016/j.semarthrit.2018.03.001. PMID: 29622343. PMCID: PMC6131088.
Article38. Sengupta K, Golakoti T, Marasetti AK, Tummala T, Ravada SR, Krishnaraju AV, et al. 2009; Inhibition of TNFα production and blocking of mitogen-activated protein kinase/NFκB activation in lipopolysaccharide-induced THP-1 human monocytes by 3-O-acetyl-11-keto-β-boswellic acid. J Food Lipids. 16:325–44. DOI: 10.1111/j.1745-4522.2009.01150.x.
Article39. Yu G, Xiang W, Zhang T, Zeng L, Yang K, Li J. 2020; Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: a systematic review and meta-analysis. BMC Complement Med Ther. 20:225. DOI: 10.1186/s12906-020-02985-6. PMID: 32680575. PMCID: PMC7368679.
Article40. Jhun J, Na HS, Cho KH, Kim J, Moon YM, Lee SY, et al. 2021; A green-lipped mussel reduces pain behavior and chondrocyte inflammation and attenuated experimental osteoarthritis progression. PLoS One. 16:e0259130. DOI: 10.1371/journal.pone.0259130. PMID: 34855756. PMCID: PMC8638931.
Article41. Siriarchavatana P, Kruger MC, Miller MR, Tian HS, Wolber FM. 2019; The preventive effects of Greenshell mussel (Perna canaliculus) on early-stage metabolic osteoarthritis in rats with diet-induced obesity. Nutrients. 11:1601. DOI: 10.3390/nu11071601. PMID: 31311115. PMCID: PMC6683089.
Article42. Abshirini M, Coad J, Wolber FM, von Hurst P, Miller MR, Tian HS, et al. 2021; Green-lipped (greenshell™) mussel (Perna canaliculus) extract supplementation in treatment of osteoarthritis: a systematic review. Inflammopharmacology. 29:925–38. DOI: 10.1007/s10787-021-00801-2. PMID: 33738701. PMCID: PMC8298224.
Article43. Abshirini M, Coad J, Wolber FM, von Hurst P, Miller MR, Tian HS, et al. 2022; Effects of Greenshell™ mussel intervention on biomarkers of cartilage metabolism, inflammatory markers and joint symptoms in overweight/obese postmenopausal women: a randomized, double-blind, and placebo-controlled trial. Front Med (Lausanne). 9:1063336. DOI: 10.3389/fmed.2022.1063336. PMID: 36544504. PMCID: PMC9760926.
Article44. Stebbings S, Gray A, Schneiders AG, Sansom A. 2017; A randomized double-blind placebo-controlled trial to investigate the effectiveness and safety of a novel green-lipped mussel extract -BioLex® -for managing pain in moderate to severe osteoarthritis of the hip and knee. BMC Complement Altern Med. 17:416. DOI: 10.1186/s12906-017-1907-9. PMID: 28830491. PMCID: PMC5568208.
Article45. Winther K, Apel K, Thamsborg G. 2005; A powder made from seeds and shells of a rose-hip subspecies (Rosa canina) reduces symptoms of knee and hip osteoarthritis: a randomized, double-blind, placebo controlled clinical trial. Scand J Rheumatol. 34:302–8. DOI: 10.1080/03009740510018624. PMID: 16195164.46. Warholm O, Skaar S, Hedman E, Mølmen HM, Eik L. 2003; The effects of a standardized herbal remedy made from a subtype of Rosa canina in patients with osteoarthritis: a double-blind, randomized, placebo-controlled clinical trial. Curr Ther Res Clin Exp. 64:21–31. DOI: 10.1016/S0011-393X(03)00004-3. PMID: 24944354.
Article47. Schwager J, Richard N, Schoop R, Wolfram S. 2014; A novel rose hip preparation with enhanced anti-inflammatory and chondroprotective effects. Mediators Inflamm. 2014:105710. DOI: 10.1155/2014/105710. PMID: 25371599. PMCID: PMC4211164.
Article48. Kocaadam B, Şanlier N. 2017; Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit Rev Food Sci Nutr. 57:2889–95. DOI: 10.1080/10408398.2015.1077195. PMID: 26528921.49. Soleimani V, Sahebkar A, Hosseinzadeh H. 2018; Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: review. Phytother Res. 32:985–95. DOI: 10.1002/ptr.6054. PMID: 29480523.50. Liczbiński P, Michałowicz J, Bukowska B. 2020; Molecular mechanism of curcumin action in signaling pathways: review of the latest research. Phytother Res. 34:1992–2005. DOI: 10.1002/ptr.6663. PMID: 32141677.
Article51. Zeng L, Yu G, Hao W, Yang K, Chen H. 2021; The efficacy and safety of Curcuma longa extract and curcumin supplements on osteoarthritis: a systematic review and meta-analysis. Biosci Rep. 41:BSR20210817. DOI: 10.1042/BSR20210817. PMID: 34017975. PMCID: PMC8202067.
Article52. Lau KM, Yue GG, Chan YY, Kwok HF, Gao S, Wong CW, et al. 2019; A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin Med. 14:25. DOI: 10.1186/s13020-019-0250-0. PMID: 31388349. PMCID: PMC6670126.53. Yoon TJ, Yoo YC, Lee SW, Shin KS, Choi WH, Hwang SH, et al. 2004; Anti-metastatic activity of Acanthopanax senticosus extract and its possible immunological mechanism of action. J Ethnopharmacol. 93:247–53. DOI: 10.1016/j.jep.2004.03.052. PMID: 15234760.
Article54. Lin J, Li X, Qi W, Yan Y, Chen K, Xue X, et al. 2018; Isofraxidin inhibits interleukin-1β induced inflammatory response in human osteoarthritis chondrocytes. Int Immunopharmacol. 64:238–45. DOI: 10.1016/j.intimp.2018.09.003. PMID: 30205322.
Article55. He X, Wang X, Fang J, Chang Y, Ning N, Guo H, et al. 2017; The genus Achyranthes: a review on traditional uses, phytochemistry, and pharmacological activities. J Ethnopharmacol. 203:260–78. DOI: 10.1016/j.jep.2017.03.035. PMID: 28347832.56. Kim D, Lee D, Oh D, Jeong HC, Lee SJ, Sohn J, et al. 2020; A mixture containing fermented Achyranthes japonica Nakai ameliorates osteoarthritis in knee joints of monoiodoacetate-injected rats. J Med Food. 23:811–7. DOI: 10.1089/jmf.2019.4552. PMID: 32614635.57. Kang HS, Lee HS, Yu HJ, Jang SH, Seo Y, Cho HY, et al. 2017; Effect of fermented Achyranthes japonica (Miq.) Nakai extract on osteoarthritis. Korean J Food Sci Technol. 49:104–9. DOI: 10.9721/KJFST.2017.49.1.104.
Article58. Chen Z, Wu G, Zheng R. 2020; A systematic pharmacology and in vitro study to identify the role of the active compounds of Achyranthes bidentata in the treatment of osteoarthritis. Med Sci Monit. 26:e925545. DOI: 10.12659/MSM.925545.
Article59. Hong JM, Shin JK, Kim JY, Jang MJ, Park SK, Lee JH, et al. 2018; BST106 protects against cartilage damage by inhibition of apoptosis and enhancement of autophagy in osteoarthritic rats. Biol Pharm Bull. 41:1257–68. DOI: 10.1248/bpb.b18-00207. PMID: 29794403.
Article60. Lee JH, Seo JY, Ko NY, Chang SH, Her E, Park T, et al. 2004; Inhibitory activity of Chrysanthemi sibirici herba extract on RBL-2H3 mast cells and compound 48/80-induced anaphylaxis. J Ethnopharmacol. 95:425–30. DOI: 10.1016/j.jep.2004.08.023. PMID: 15507370.
Article61. Byun JH, Choi CW, Jang MJ, Lim SH, Han HJ, Choung SY. 2020; Anti-osteoarthritic mechanisms of Chrysanthemum zawadskii var. latilobum in MIA-induced osteoarthritic rats and interleukin-1β-induced SW1353 human chondrocytes. Medicina (Kaunas). 56:685. DOI: 10.3390/medicina56120685. PMID: 33321982. PMCID: PMC7762971.
Article62. Ha JK, Kim JS, Kim JY, Yun JB, Kim YY, Chung KS. 2021; Efficacy of GCWB106 (Chrysanthemum zawadskii var. latilobum extract) in osteoarthritis of the knee: a 12-week randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 100:e26542. DOI: 10.1097/MD.0000000000026542. PMID: 34190191. PMCID: PMC8257904.63. Koo HJ, Yoon WJ, Sohn EH, Ham YM, Jang SA, Kwon JE, et al. 2014; The analgesic and anti-inflammatory effects of Litsea japonica fruit are mediated via suppression of NF-κB and JNK/p38 MAPK activation. Int Immunopharmacol. 22:84–97. DOI: 10.1016/j.intimp.2014.06.007. PMID: 24968348.
Article64. Jeong YJ, Kim I, Cho JH, Park DW, Kwon JE, Jung MW, et al. 2015; Anti-osteoarthritic effects of the Litsea japonica fruit in a rat model of osteoarthritis induced by monosodium iodoacetate. PLoS One. 10:e0134856. DOI: 10.1371/journal.pone.0134856. PMID: 26244981. PMCID: PMC4526681.
Article65. Ahn Y, Kwon O, Kim EA, Yoon WJ, Kim JH, Kim JY. 2017; Randomized double-blind placebo-controlled study of the efficacy of Litsea japonica fruit extract in subjects with mild to moderate knee osteoarthritis. J Funct Foods. 34:304–10. DOI: 10.1016/j.jff.2017.05.005.66. Chen J, Huang L, Liao X. 2023; Protective effects of ginseng and ginsenosides in the development of osteoarthritis (Review). Exp Ther Med. 26:465. DOI: 10.3892/etm.2023.12164. PMID: 37664679. PMCID: PMC10468808.
Article67. Cheng W, Jing J, Wang Z, Wu D, Huang Y. 2017; Chondroprotective effects of ginsenoside Rg1 in human osteoarthritis chondrocytes and a rat model of anterior cruciate ligament transection. Nutrients. 9:263. DOI: 10.3390/nu9030263. PMID: 28287423. PMCID: PMC5372926.
Article68. Cheng W, Wu D, Zuo Q, Wang Z, Fan W. 2013; Ginsenoside Rb1 prevents interleukin-1 beta induced inflammation and apoptosis in human articular chondrocytes. Int Orthop. 37:2065–70. DOI: 10.1007/s00264-013-1990-6. PMID: 23835558. PMCID: PMC3779573.
Article69. Chen M, Zhou S, Liu L, Wen Y, Chen L. 2021; Notoginsenoside R1 alleviates the inflammation of osteoarthritis by activating the Nrf2/HO-1 signalling pathway in vitro and in vivo. J Funct Foods. 85:104666. DOI: 10.1016/j.jff.2021.104666.
Article70. Kim HI, Chon SJ, Seon KE, Seo SK, Choi YR. 2021; Clinical effects of Korean red ginseng in postmenopausal women with hand osteoarthritis: a double-blind, randomized controlled trial. Front Pharmacol. 12:745568. DOI: 10.3389/fphar.2021.745568. PMID: 34858175. PMCID: PMC8630590.
Article