J Liver Cancer.
2024 Mar;24(1):81-91. 10.17998/jlc.2023.12.25.
Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Liver Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Cancer Research Institute, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2553820
- DOI: http://doi.org/10.17998/jlc.2023.12.25
Abstract
- Background
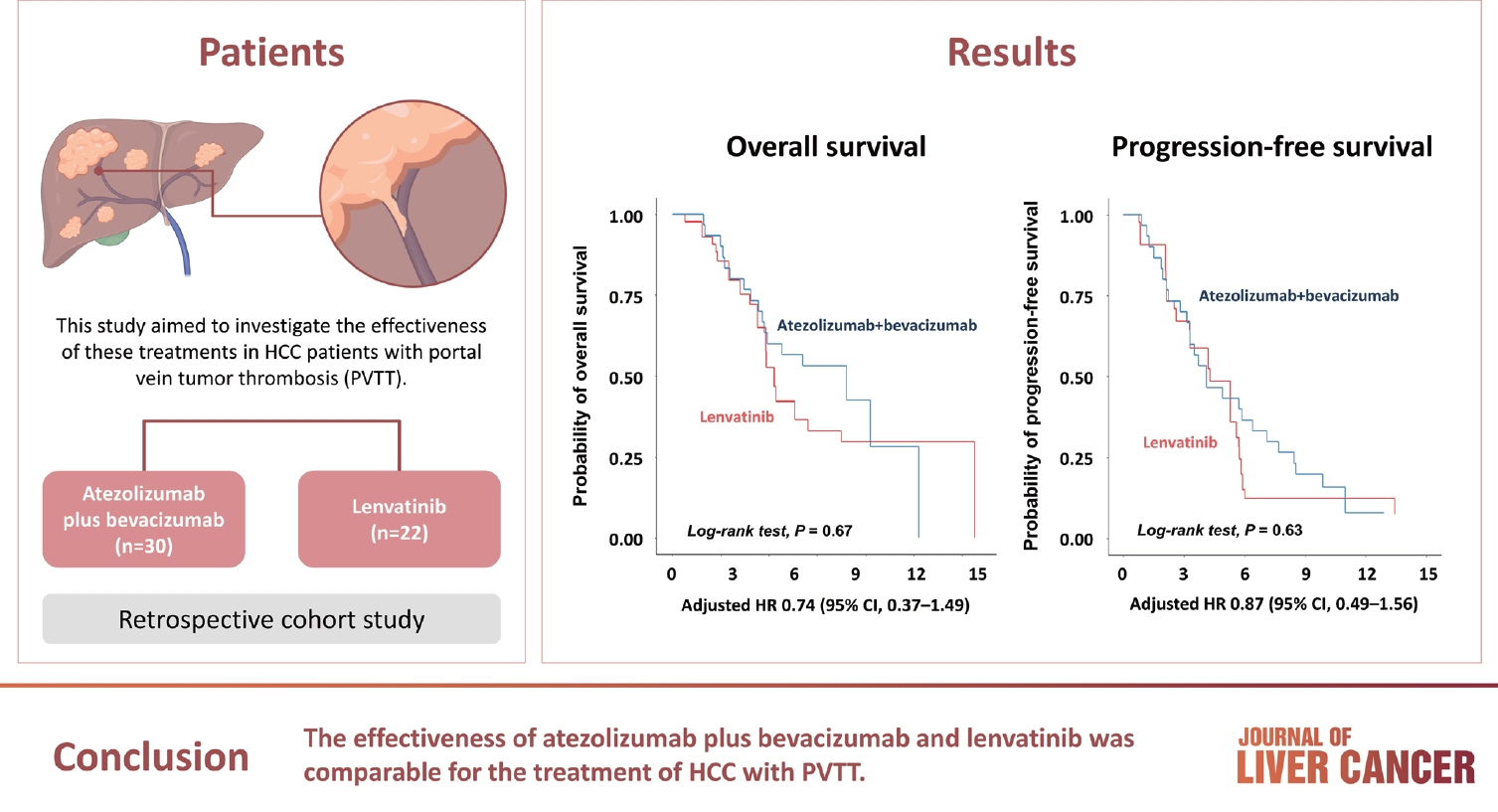
/Aim: Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
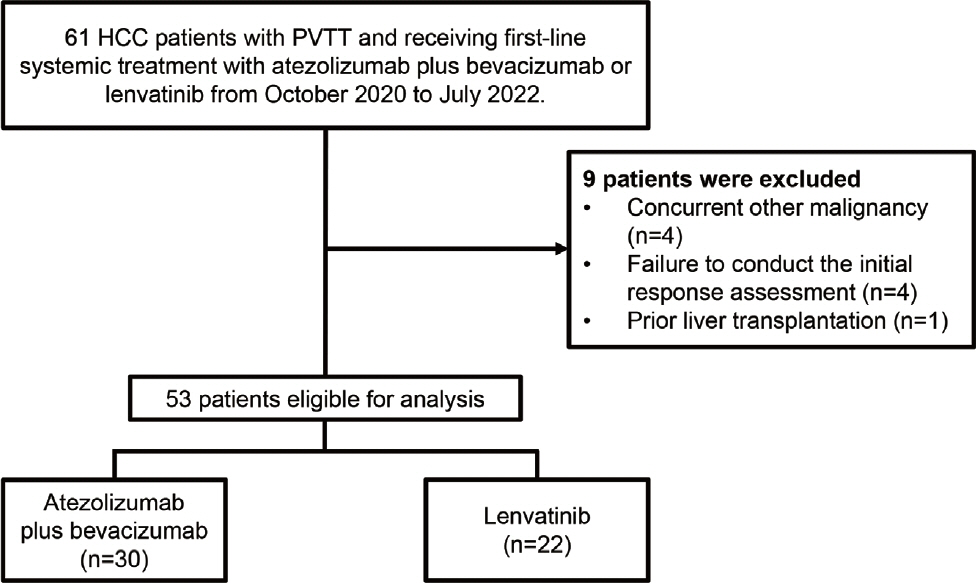
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
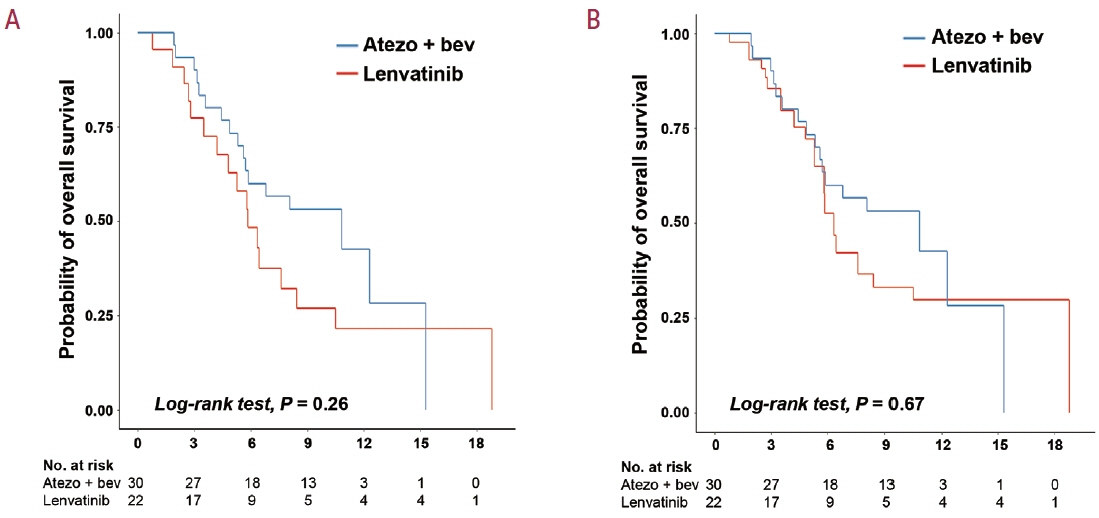
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.
Figure
Reference
-
References
1. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021; 73 Suppl 1:4–13.
Article2. Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 2022; 1:EVIDoa2100070.
Article3. Cho Y, Han J, Kim W. Recent advances and future directions in immunotherapeutics for hepatocellular carcinoma. J Liver Cancer. 2019; 19:1–11.
Article4. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020; 382:1894–1905.
Article5. Yamashita T, Kudo M, Ikeda K, Izumi N, Tateishi R, Ikeda M, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020; 55:113–122.
Article6. Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/ bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel). 2022; 14:1747.
Article7. Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, et al. Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open. 2022; 7:100591.8. Jin S, Choi WM, Shim JH, Lee D, Kim KM, Lim YS, et al. Subclassification of advanced-stage hepatocellular carcinoma with macrovascular invasion: combined transarterial chemoembolization and radiotherapy as an alternative first-line treatment. J Liver Cancer. 2023; 23:177–188.
Article9. Khan AR, Wei X, Xu X. Portal vein tumor thrombosis and hepatocellular carcinoma - the changing tides. J Hepatocell Carcinoma. 2021; 8:1089–1115.
Article10. Liu PH, Huo TI, Miksad RA. Hepatocellular carcinoma with portal vein tumor involvement: best management strategies. Semin Liver Dis. 2018; 38:242–251.
Article11. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.
Article12. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.13. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. J Liver Cancer. 2023; 23:1–120.14. Nevarez NM, Yopp AC. Challenging the treatment paradigm: selecting patients for surgical management of hepatocellular carcinoma with portal vein tumor thrombus. J Hepatocell Carcinoma. 2021; 8:851–860.
Article15. Idezuki Y. General rules for recording endoscopic findings of esophagogastric varices (1991). Japanese Society for Portal Hypertension. World J Surg. 1995; 19:420–422. discussion 423.
Article16. Cunningham ME, Parastandeh-Chehr G, Cerocchi O, Wong DK, Patel K. Noninvasive predictors of high-risk varices in patients with non-cirrhotic portal hypertension. Can J Gastroenterol Hepatol. 2019; 2019:1808797.
Article17. Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016; 62:132–137.
Article18. Korean Liver Cancer Association (KLCA); National Cancer Center (NCC) Korea. 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma. Korean J Radiol. 2022; 23:1126–1240.19. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016; 35:5642–5655.
Article20. Dipasquale A, Marinello A, Santoro A. A comparison of lenvatinib versus sorafenib in the first-line treatment of unresectable hepatocellular carcinoma: selection criteria to guide physician's choice in a new therapeutic scenario. J Hepatocell Carcinoma. 2021; 8:241–251.
Article21. Kato Y, Tabata K, Kimura T, Yachie-Kinoshita A, Ozawa Y, Yamada K, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019; 14:e0212513.
Article22. Kimura T, Kato Y, Ozawa Y, Kodama K, Ito J, Ichikawa K, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018; 109:3993–4002.
Article23. Kudo M. Lenvatinib may drastically change the treatment landscape of hepatocellular carcinoma. Liver Cancer. 2018; 7:1–19.
Article24. Takase N, Koma Y, Urakawa N, Nishio M, Arai N, Akiyama H, et al. NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett. 2016; 380:47–58.
Article25. Kuwano A, Yada M, Miyazaki Y, Tanaka K, Kurosaka K, Ohishi Y, et al. Tumor‑infiltrating CD8+ T cells as a biomarker for chemotherapy efficacy in unresectable hepatocellular carcinoma. Oncol Lett. 2023; 25:259.26. Mukozu T, Nagai H, Matsui D, Mohri K, Watanabe G, Yoshimine N, et al. Adaptation of lenvatinib treatment in patients with hepatocellular carcinoma and portal vein tumor thrombosis. Cancer Chemother Pharmacol. 2022; 89:11–20.
Article27. Lee SK, Kwon JH, Lee SW, Lee HL, Kim HY, Kim CW, et al. A realworld comparative analysis of atezolizumab plus bevacizumab and transarterial chemoembolization plus radiotherapy in hepatocellular carcinoma patients with portal vein tumor thrombosis. Cancers (Basel). 2023; 15:4423.
Article28. Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014; 6:18.
Article29. Suzuki H, Iwamoto H, Shimose S, Niizeki T, Shirono T, Noda Y, et al. Case report: exacerbation of varices following atezolizumab plus bevacizumab treatment of hepatocellular carcinoma: a case series and literature review. Front Oncol. 2022; 12:948293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concurrent transarterial radioembolization and combination atezolizumab/ bevacizumab treatment of infiltrative hepatocellular carcinoma with portal vein tumor thrombosis: a case report
- Favorable response of hepatocellular carcinoma with portal vein tumor thrombosis after radiotherapy combined with atezolizumab plus bevacizumab
- Clinical features of portal vein thrombosis in hepatocellular carcinoma
- Clinical significance of the discrepancy between radiological findings and biochemical responses in atezolizumab plus bevacizumab for hepatocellular carcinoma
- Sonographic features of portal vein thrombosis