Ann Surg Treat Res.
2024 Mar;106(3):125-132. 10.4174/astr.2024.106.3.125.
Successful outcome with oral sirolimus treatment for complicated lymphatic malformations: a retrospective multicenter cohort study
- Affiliations
-
- 1Department of Surgery, Hanyang University Guri Hospital, Hanyang University School Medicine, Guri, Korea
- 2Department of Pediatric Surgery, Asan Medical Center Children’s Hospital, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 4Division of Pediatric Surgery, Department of Surgery, Chonnam National University Hospital, Gwangju, Korea
- 5Department of Pediatric Surgery, Keimyung University Dongsan Hospital, Daegu, Korea
- 6Department of Surgery, Inje University Busan Paik Hospital, Busan, Korea
- KMID: 2553377
- DOI: http://doi.org/10.4174/astr.2024.106.3.125
Abstract
- Purpose
Sirolimus has emerged as a safe and effective treatment for complicated lymphatic malformations (LMs). We aim to prove the effectiveness and safety of sirolimus as a therapeutic option for patients with complicated LMs. Methods: Fifty-eight patients with complicated LMs treated with sirolimus for at least 6 months at multicenter between July 2018 and January 2023 were enrolled. All patients were administered oral sirolimus starting at 0.8 mg/m 2 every 12 hours, with target serum concentration levels of 8–15 ng/mL. Evaluation for clinical symptoms and LMs volume on MRI were reviewed to assess treatment response and toxicities. Evaluation of disease response was divided into 3 values: complete response, partial response (significant, moderate, and modest), and progressive disease.
Results
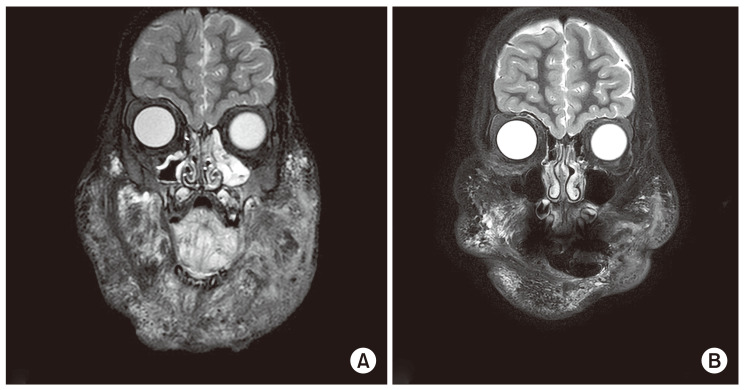
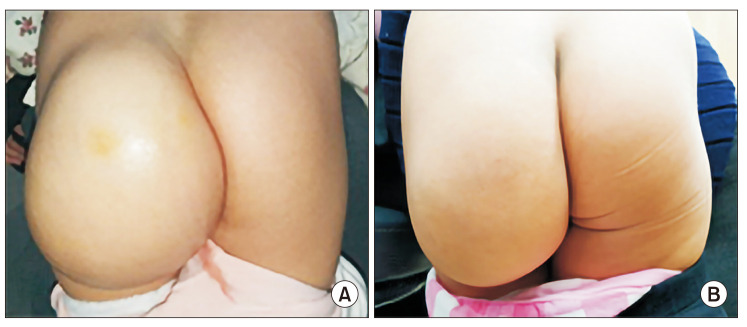
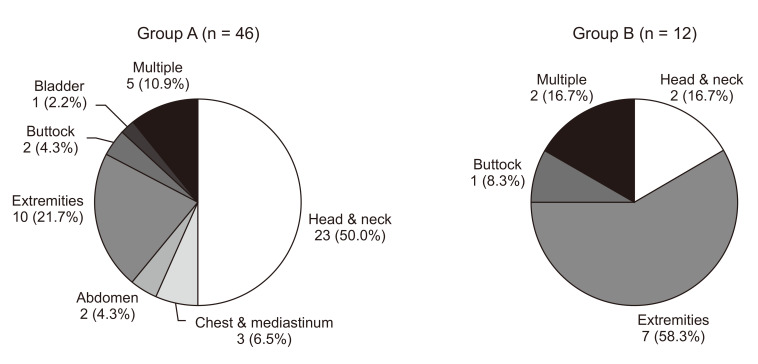
The median age at the initiation of sirolimus treatment was 6.0 years (range, 1 month–26.7 years). The median duration of treatment was 2.0 years (range, 6 months–4.4 years). The most common lesions were head and neck (25 of 58, 43.1%). Forty-six patients (79.3%) demonstrated a reduction in LMs volume on MRI or improvement of clinical symptoms including 2 complete responses. The young age group and the patients who underwent few prior therapies showed better responses. None of the patients had toxicities attributable to sirolimus with a Common Terminology Criteria for Adverse Events grade of ≥3.
Conclusion
Oral sirolimus treatment brought a successful outcome without severe adverse effects. It could be the firstline therapy, especially for the young age group of complicated LMs, and an additional option for refractory lesions that did not respond to conventional treatment.
Figure
Reference
-
1. Kennedy TL, Whitaker M, Pellitteri P, Wood WE. Cystic hygroma/lymphangioma: a rational approach to management. Laryngoscope. 2001; 111(11 Pt 1):1929–1937. PMID: 11801972.2. Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014; 124:898–904. PMID: 24590274.3. Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982; 69:412–422. PMID: 7063565.4. Bajaj Y, Hewitt R, Ifeacho S, Hartley BE. Surgical excision as primary treatment modality for extensive cervicofacial lymphatic malformations in children. Int J Pediatr Otorhinolaryngol. 2011; 75:673–677. PMID: 21419500.5. Heit JJ, Do HM, Prestigiacomo CJ, Delgado-Almandoz JA, English J, Gandhi CD, et al. Guidelines and parameters: percutaneous sclerotherapy for the treatment of head and neck venous and lymphatic malformations. J Neurointerv Surg. 2017; 9:611–617. PMID: 26801946.6. Berg EE, Sobol SE, Jacobs I. Laryngeal obstruction by cervical and endolaryngeal lymphatic malformations in children: proposed staging system and review of treatment. Ann Otol Rhinol Laryngol. 2013; 122:575–581. PMID: 24224401.7. Kushner BH, LaQuaglia MP, Wollner N, Meyers PA, Lindsley KL, Ghavimi F, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996; 14:1526–1531. PMID: 8622067.8. Triana P, Dore M, Cerezo VN, Cervantes M, Sánchez AV, Ferrero MM, et al. Sirolimus in the treatment of vascular anomalies. Eur J Pediatr Surg. 2017; 27:86–90. PMID: 27723921.9. de Vasconcellos JF, Laranjeira AB, Zanchin NI, Otubo R, Vaz TH, Cardoso AA, et al. Increased CCL2 and IL-8 in the bone marrow microenvironment in acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011; 56:568–577. PMID: 21298741.10. Tian R, Liang Y, Zhang W, Wang J, Shan Y, Gao H, et al. Effectiveness of sirolimus in the treatment of complex lymphatic malformations: single center report of 56 cases. J Pediatr Surg. 2020; 55:2454–2458. PMID: 32044101.11. Strychowsky JE, Rahbar R, O’Hare MJ, Irace AL, Padua H, Trenor CC 3rd. Sirolimus as treatment for 19 patients with refractory cervicofacial lymphatic malformation. Laryngoscope. 2018; 128:269–276. PMID: 28782106.12. Alemi AS, Rosbe KW, Chan DK, Meyer AK. Airway response to sirolimus therapy for the treatment of complex pediatric lymphatic malformations. Int J Pediatr Otorhinolaryngol. 2015; 79:2466–2469. PMID: 26549380.13. Lackner H, Karastaneva A, Schwinger W, Benesch M, Sovinz P, Seidel M, et al. Sirolimus for the treatment of children with various complicated vascular anomalies. Eur J Pediatr. 2015; 174:1579–1584. PMID: 26040705.14. Teng JM, Hammill A, Martini J, Treat J. Sirolimus in the treatment of microcystic lymphatic malformations: a systematic review. Lymphat Res Biol. 2023; 21:101–110. PMID: 35852876.15. Cho YJ, Kwon H, Kwon YJ, Kim SC, Kim DY, Namgoong JM. Effects of sirolimus in the treatment of unresectable infantile hemangioma and vascular malformations in children: a single-center experience. J Vasc Surg Venous Lymphat Disord. 2021; 9:1488–1494. PMID: 33836285.16. Savarese D. Common terminology criteria for adverse events. UpToDate. 2013. p. 1–9.17. Pupulim LF, Ronot M, Paradis V, Chemouny S, Vilgrain V. Volumetric measurement of hepatic tumors: accuracy of manual contouring using CT with volumetric pathology as the reference method. Diagn Interv Imaging. 2018; 99:83–89. PMID: 29221936.18. Soyer P, Roche A, Elias D, Levesque M. Hepatic metastases from colorectal cancer: influence of hepatic volumetric analysis on surgical decision making. Radiology. 1992; 184:695–697. PMID: 1509051.19. Adams DM, Trenor CC 3rd, Hammill AM, Vinks AA, Patel MN, Chaudry G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. 2016; 137:e20153257. PMID: 26783326.20. Kulungowski AM, Patel M. Lymphatic malformations. Semin Pediatr Surg. 2020; 29:150971. PMID: 33069296.21. Dasgupta R, Adams D, Elluru R, Wentzel MS, Azizkhan RG. Noninterventional treatment of selected head and neck lymphatic malformations. J Pediatr Surg. 2008; 43:869–873. PMID: 18485956.22. Savas JA, Ledon J, Franca K, Chacon A, Zaiac M, Nouri K. Carbon dioxide laser for the treatment of microcystic lymphatic malformations (lymphangioma circumscriptum): a systematic review. Dermatol Surg. 2013; 39:1147–1157. PMID: 23607875.23. Fliegelman LJ, Friedland D, Brandwein M, Rothschild M. Lymphatic malformation: predictive factors for recurrence. Otolaryngol Head Neck Surg. 2000; 123:706–710. PMID: 11112962.24. Tu JH, Do HM, Patel V, Yeom KW, Teng JM. Sclerotherapy for lymphatic malformations of the head and neck in the pediatric population. J Neurointerv Surg. 2017; 9:1023–1026. PMID: 27707871.25. Ogita S, Tsuto T, Tokiwa K, Takahashi T. Intracystic injection of OK-432: a new sclerosing therapy for cystic hygroma in children. Br J Surg. 1987; 74:690–691. PMID: 3651773.26. Patel V, Tu JH, Teng JM. Natural history of lymphatic malformation progression in children: a retrospective study. J Clin Exp Dermatol Res. 2017; 8:1000424.27. Riechelmann H, Muehlfay G, Keck T, Mattfeldt T, Rettinger G. Total, subtotal, and partial surgical removal of cervicofacial lymphangiomas. Arch Otolaryngol Head Neck Surg. 1999; 125:643–648. PMID: 10367920.28. Yesil S, Bozkurt C, Tanyildiz HG, Tekgunduz SA, Candir MO, Toprak S, et al. Successful treatment of macroglossia due to lymphatic malformation with sirolimus. Ann Otol Rhinol Laryngol. 2015; 124:820–823. PMID: 25902840.29. Pandey V, Tiwari P, Sharma SP, Kumar R, Panigrahi P, Singh OP, et al. Development of a biomarker of efficacy in second-line treatment for lymphangioma of the tongue: a pilot study. Br J Oral Maxillofac Surg. 2019; 57:1137–1142. PMID: 31727434.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lymphatic Malformations of the Orbit
- Radiofrequency Ablation of Microcystic Lymphatic Malformation in the Oral Cavity: 2 Case Studies
- Sirolimus therapy for fetal cardiac rhabdomyoma in a pregnant woman with tuberous sclerosis
- Sirolimus Treatment of Refractory Infantile Hemangiomatosis With Brain Involvement
- Abdominal Lymphatic Malformation in Children