Ann Pediatr Endocrinol Metab.
2024 Feb;29(1):19-28. 10.6065/apem.2346136.068.
Association of maternal insulin resistance with neonatal insulin resistance and body composition/size: a prospective cohort study in a sub-Saharan African population
- Affiliations
-
- 1Department of Paediatrics and Child Health, Lagos State University College of Medicine/Lagos State University Teaching Hospital, Lagos, Nigeria
- 2Department of Paediatrics, Lagos State University Teaching Hospital, Lagos, Nigeria
- 3Department of Chemical Pathology, Lagos State University College of Medicine/Lagos State University Teaching Hospital, Lagos, Nigeria
- 4Department of Obstetrics and Gynaecology, Lagos State University College of Medicine/Lagos State University Teaching Hospital, Lagos, Nigeria
- KMID: 2553014
- DOI: http://doi.org/10.6065/apem.2346136.068
Abstract
- Purpose
We prospectively evaluated the association of the insulin resistance of third-trimester Nigerian pregnant women with their newborn infants' insulin resistance and birth size. Pregnancy-associated insulin resistance (IR), often assessed with homeostatic model assessment of IR (HOMA-IR), is associated, especially among women with gestational diabetes (GDM), with abnormal neonatal birth size and body composition, predisposing the baby to metabolic disorders like diabetes and obesity. The associations of maternal IR with neonatal IR, birth size and body composition are less studied in nondiabetic pregnant women, especially in sub-Saharan settings like Nigeria.
Methods
We originally recruited 401 third trimester, nondiabetic pregnant women to a prospective cohort study, followed up until birth. Blood samples of mothers and babies were obtained, respectively, at recruitment and within 24 hours postbirth for fasting serum glucose (FSG) and insulin (FSI) assays, and HOMA-IR was calculated as [(FSI × FSG)/22.5)].
Results
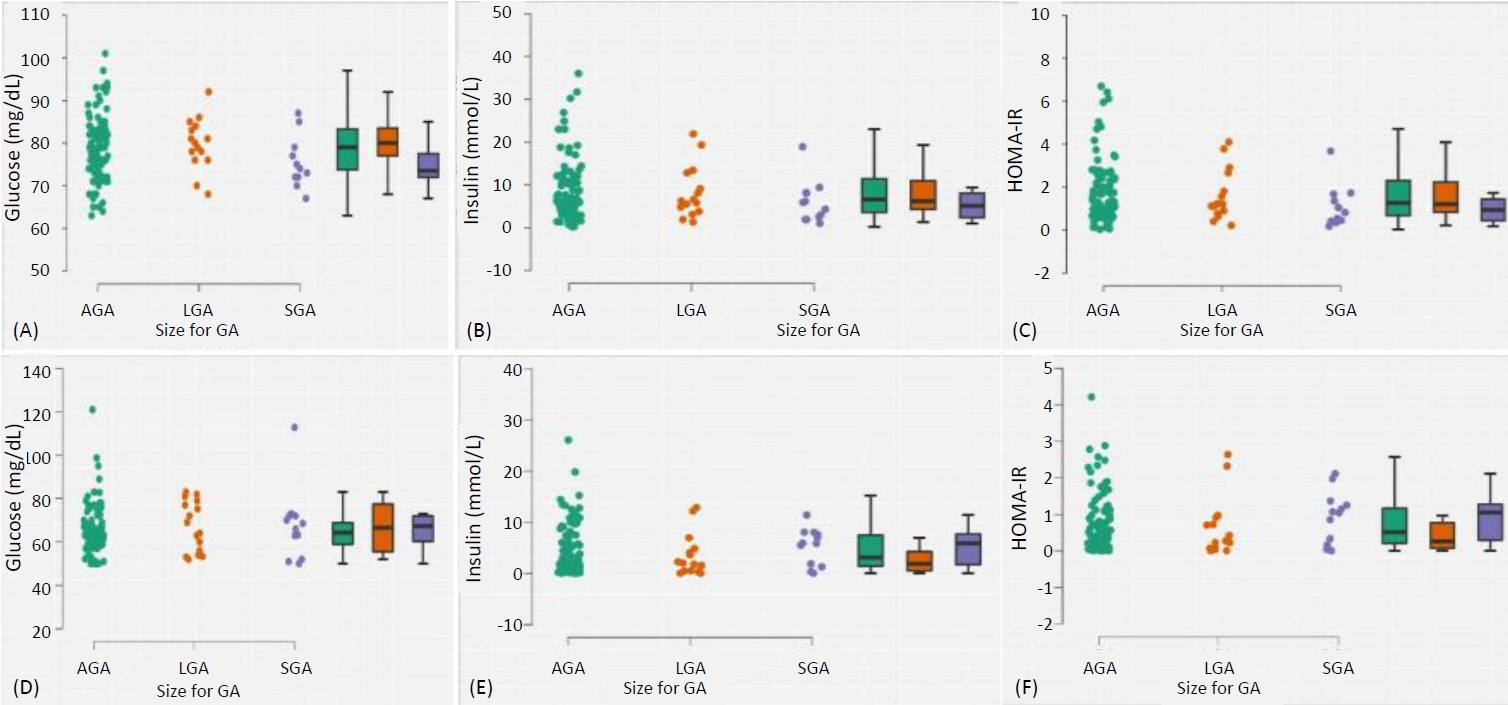
Complete data for 150 mother-baby dyads was analysed: the mothers, with a mean (standard deviation [SD]) age of 31.6 (4.5) years, had live births at a mean (SD) gestational age of 39.2 weeks. The proportions of infants with wasting, stunting, impaired fetal growth (either wasting or stunted), small-for-gestation-age, large-for-gestational-age, low birthweight, and macrosomia were 4.2% (95% confidence interval, 1.1–10.3), 19.7% (12.9–28.0), 23.1% (15.8–31.8), 10.1% (5.3–17.0), 12.6% (7.2–19.9), 0.8% (0.02–4.5), and 5.0% (1.8–10.5), respectively. Maternal HOMA-IR was not associated with neonatal HOMA-IR (p=0.837), birth weight (p=0.416) or body composition measured with weight-length ratio (p=0.524), but birth weight was independently predicted by maternal weight (p=0.006), body mass index (p=0.001), and parity (p=0.012).
Conclusion
In this nondiabetic/non-GDM cohort, maternal HOMA-IR was not associated with neonatal IR, body size or body composition. Larger studies are required to confirm these findings, with addi-tional inclusion of mothers with hyperglycaemia for comparison.
Keyword
Figure
Reference
-
References
1. Barbour L, McCurdy C, Hernandez T, Kirwan J, Catalano P, Friedman J. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007; 30 Suppl 2:S112–9.
Article2. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017; 356:j1.
Article3. Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005; 115:e500–3.
Article4. Schwartz B, Jacobs DR Jr, Moran A, Steinberger J, Hong CP, Sinaiko AR. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008; 31:783–8.5. Whincup PH, Gilg JA, Papacosta O, Seymour C, Miller GJ, Alberti KGMM, et al. Early evi-dence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ. 2002; 324:635.
Article6. Yao L, Herlea-Pana O, Heuser-Baker J, Chen Y, Barlic-Dicen J. Roles of the chemokine sys-tem in development of obesity, insulin resistance, and cardiovascular disease. J Immunol Res. 2014; 2014:181450.
Article7. Patel TP, Rawal K, Bagchi AK, Akolkar G, Bernardes N, Dias DDS, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016; 21:11–23.8. Walsh JM, McGowan CA, Mahony RM, Foley ME, McAuliffe FM. Obstetric and metabolic implications of excessive gestational weight gain in pregnancy. Obesity. 2014; 22:1594–600.
Article9. Akinola IJ, Oyenusi EE, Odusote OA, Oduwole AO, Njokanma FO. Insulin resistance profile of apparently healthy term neonates in Lagos, Nigeria. J Clin Neonatol. 2018; 7:71–4.10. Akinmola OO, Okusanya BO, Olorunfemi G, Okpara HC, Azinge EC. Fetal macrosomia, fetal insulin, and insulin-like growth factor-1 among neonates in Lagos, Nigeria: a casecontrol study. PLoS One. 2022; 17:e0266314.11. Naseh A, Nourbakhsh S, Tohidi M, Sarkhail P, Najafian B, Azizi F. Associations between anthropometric characteristics and insulin markers in mothers and their neonates and with ne-onate's birth weight: an observational cohort study. Turk J Pediatr. 2017; 59:625–35.
Article12. Drozdz D, Alvarez-Pitti J, Wójcik M, Borghi C, Gabbianelli R, Mazur A, et al. Obesity and cardiometabolic risk factors: from childhood to adulthood. Nutrients. 2021; 13:4176.
Article13. Lurbe E, Ingelfinger J. Developmental and early life origins of cardiometabolic risk factors. Hypertension. 2021; 77:308–18.
Article14. Martín-Calvo N, Goni L, Tur JA, Martínez JA. Low birth weight and small for gestational age are associated with complications of childhood and adolescence obesity: systematic re-view and meta-analysis. Obes Rev. 2022; 23 Suppl 1:e13380.15. Pintaudi B, Fresa R, Dalfrà M, Dodesini AR, Vitacolonna E, Tumminia A, et al. The risk stratification of adverse neonatal outcomes in women with gestational diabetes (STRONG) study. Acta Diabetol. 2018; 55:1261–73.
Article16. Malaza N, Masete M, Adam S, Dias S, Nyawo T, Pheiffer C. A systematic review to compare adverse pregnancy outcomes in women with pregestational diabetes and gestational diabetes. Int J Environ Res Public Health. 2022; 19:10846.17. Timur BB, Timur H, Tokmak A, Isik H, Eyi EGY. The influence of maternal obesity on preg-nancy complications and neonatal outcomes in diabetic and nondiabetic women. Geburtshilfe Frauenheilkd. 2018; 78:400–6.
Article18. Yamashita H, Yasuhi I, Fukuda M, Kugishima Y, Yamauchi Y, Kuzume A, et al. The associa-tion between maternal insulin resistance in mid-pregnancy and neonatal birthweight in un-complicated pregnancies. Endocr J. 2014; 61:1019–24.
Article19. Chionuma JO, Akinola IJ, Dada AO, Ubuane PO, Kuku-Kuye TO, Olalere FD. Profile of insulin resistance of pregnant women at late third trimester in Nigeria: a descriptive cross-sectional report. Niger J Clin Pract. 2022; 25:1736–44.20. Imoh LC, Ocheke AN. Correlation between maternal weight and insulin resistance in second half of pregnancy. Niger Med J J Niger Med Assoc. 2014; 55:465–8.21. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a World Health Organization Guideline. Diabetes Res Clin Pract. 2014; 103:341–63.22. Masoodian SM, Omidifar A, Moradkhani S, Asiabanha M, Khoshmirsafa M. HOMA-IR mean values in healthy individuals: a population-based study in iranian subjects. J Diabetes Metab Disord. 2023; 22:219–24.23. Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003; 26:3320–5.24. Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet Lond Engl. 2014; 384:857–68.25. Victora CG, Villar J, Barros FC, Ismail LC, Chumlea C, Papageorghiou AT, et al. Anthropo-metric characterization of impaired fetal growth: risk factors for and prognosis of newborns with stunting or wasting. JAMA Pediatr. 2015; 169:e151431.26. Villar J, Puglia FA, Fenton TR, Cheikh Ismail L, Staines-Urias E, Giuliani F, et al. Body composition at birth and its relationship with neonatal anthropometric ratios: the newborn body composition study of the INTERGROWTH-21st project. Pediatr Res. 2017; 82:305–16.27. Villar J, Giuliani F, Fenton TR, Ohuma EO, Ismail LC, Kennedy SH. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016; 387:844–5.28. Mokuolu OA, Adesiyun OO, Suleiman MB, Bello M. Intrauterine growth standards: a cross-sectional study in a population of Nigerian newborns. Pediatr Rep. 2012; 4:e29.29. Wang Q, Huang R, Yu B, Cao F, Wang H, Zhang M, et al. Higher fetal insulin resistance in Chinese pregnant women with gestational diabetes mellitus and correlation with maternal in-sulin resistance. PLoS One. 2013; 8:e59845.30. Maric T, Kanu C, Johnson MR, Savvidou MD. Maternal, neonatal insulin resistance and ne-onatal anthropometrics in pregnancies following bariatric surgery. Metabolism. 2019; 97:25–31.
Article31. Bomba-Opon DA, Wielgos M, Horosz E, Bartkowiak R, Szymusik I, Mazanowski N, et al. Maternal plasma cytokines concentrations and insulin resistance in first trimester in relation to fetal growth. Neuro Endocrinol Lett. 2009; 30:729–32.32. Wang D, Zhu L, Zhang S, Wu X, Wang X, Lv Q, et al. Predictive macrosomia birthweight thresholds for adverse maternal and neonatal outcomes. J Matern Fetal Neonatal Med. 2016; 29:3745–50.33. Lin J, Jin H, Chen L. Associations between insulin resistance and adverse pregnancy out-comes in women with gestational diabetes mellitus: a retrospective study. BMC Pregnancy Childbirth. 2021; 21:526.34. Simental-Mendía LE, Castañeda-Chacón A, Rodríguez-Morán M, Guerrero-Romero F. Birth-weight, insulin levels, and HOMA-IR in newborns at term. BMC Pediatr. 2012; 12:94.35. Lewandowska M. The role of maternal weight in the hierarchy of macrosomia predictors; overall effect of analysis of three prediction indicators. Nutrients. 2021; 13:801.36. Shapiro ALB, Schmiege SJ, Brinton JT, Glueck D, Crume TL, Friedman JE, et al. Testing the fuel-mediated hypothesis: maternal insulin resistance and glucose mediate the association be-tween maternal and neonatal adiposity, the Healthy Start study. Diabetologia. 2015; 58:937–41.37. Sandler V, Reisetter AC, Bain JR, Muehlbauer MJ, Nodzenski M, Stevens RD, et al. Associa-tions of maternal BMI and insulin resistance with the maternal metabolome and newborn out-comes. Diabetologia. 2017; 60:518–30.38. Thompson WD, Beaumont RN, Kuang A, Warrington NM, Ji Y, Tyrrell J, et al. Higher ma-ternal adiposity reduces offspring birthweight if associated with a metabolically favourable profile. Diabetologia. 2021; 64:2790–802.39. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. 2019; 2019:5320156.40. Lima RA, Desoye G, Simmons D, Devlieger R, Galjaard S, Corcoy R, et al. The importance of maternal insulin resistance throughout pregnancy on neonatal adiposity. Paediatr Perinat Epidemiol. 2021; 35:83–91.41. Walsh JM, Segurado R, Mahony RM, Foley ME, McAuliffe FM. The effects of fetal gender on maternal and fetal insulin resistance. PLoS One. 2015; 10:e0137215.42. Mitanchez D, Jacqueminet S, Nizard J, Tanguy ML, Ciangura C, Lacorte JM, et al. Effect of maternal obesity on birthweight and neonatal fat mass: a prospective clinical trial. PLoS One. 2017; 12:e0181307.