Endocrinol Metab.
2024 Feb;39(1):109-126. 10.3803/EnM.2023.1839.
Efficacy and Safety of Omarigliptin, a Novel Once-Weekly Dipeptidyl Peptidase-4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Endocrinology, Mymensingh Medical College, Mymensingh, Bangladesh
- 2Department of Medicine, Army Medical College Cumilla, Cumilla, Bangladesh
- 3Department of Endocrinology, Rangpur Medical College, Rangpur, Bangladesh
- 4Department of Endocrinology, Center for Endocrinology, Diabetes, Arthritis and Rheumatism (CEDAR) Superspeciality Healthcare, New Delhi, India
- 5Department of Endocrinology, Bangabandhu Sheikh Mujib Medical University, Dhaka, Bangladesh
- KMID: 2552803
- DOI: http://doi.org/10.3803/EnM.2023.1839
Abstract
- Background
No recent meta-analysis has holistically analyzed and summarized the efficacy and safety of omarigliptin in type 2 diabetes mellitus (T2DM). We conducted a meta-analysis to address this knowledge gap.
Methods
Electronic databases were searched to identify randomized controlled trials (RCTs) that included patients with T2DM who received omarigliptin in the intervention arm. The control arm consisted of either a placebo (passive control group [PCG]) or an active comparator (active control group [ACG]). The primary outcome assessed was changes in hemoglobin A1c (HbA1c), while secondary outcomes included variations in glucose levels, achievement of glycemic targets, adverse events (AEs), and hypoglycemic events.
Results
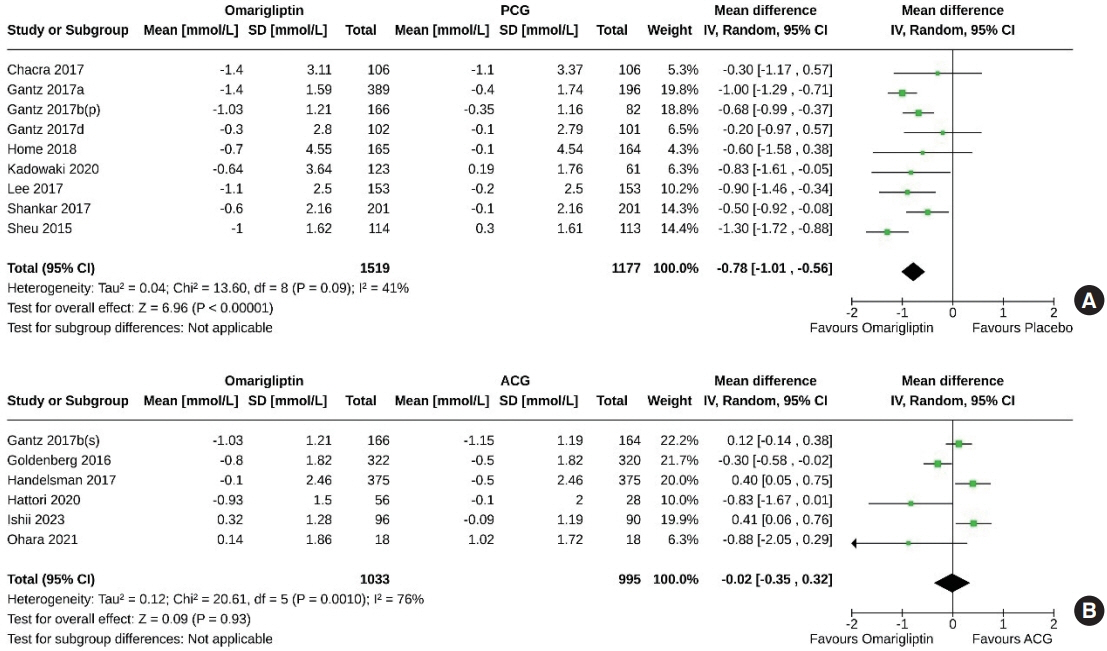
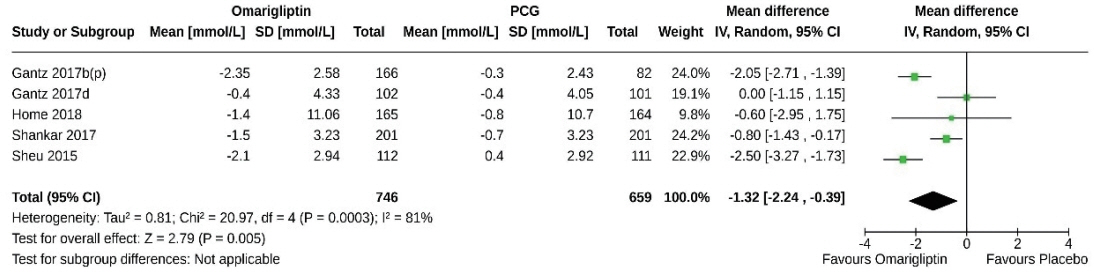
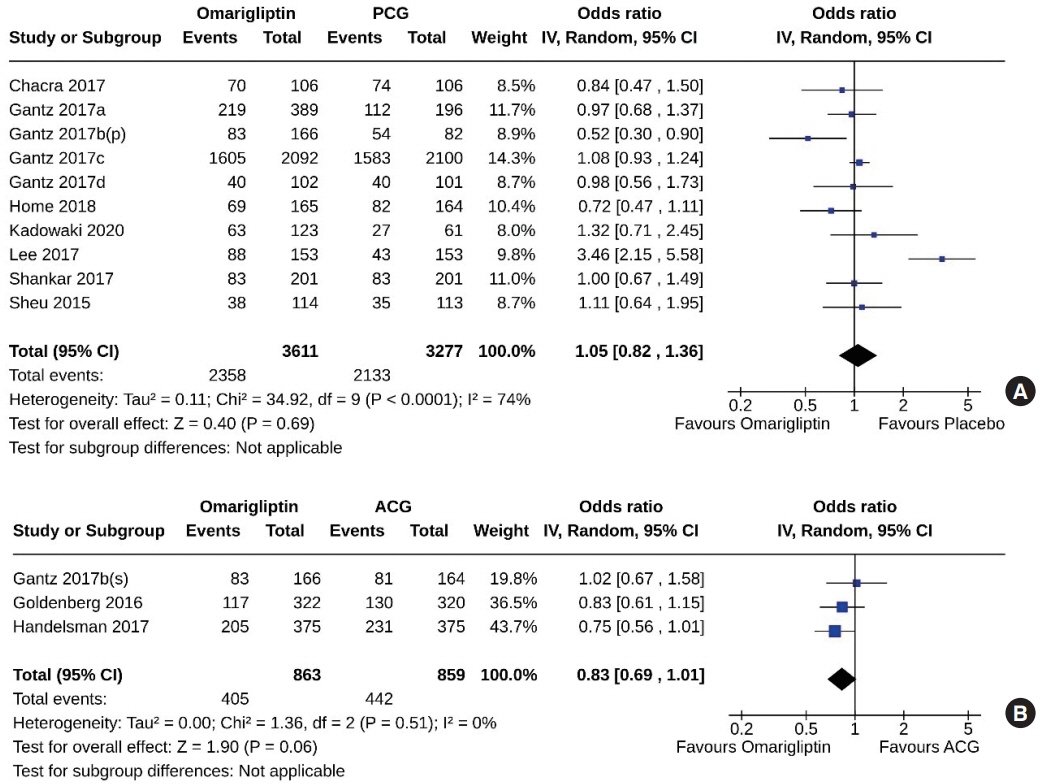
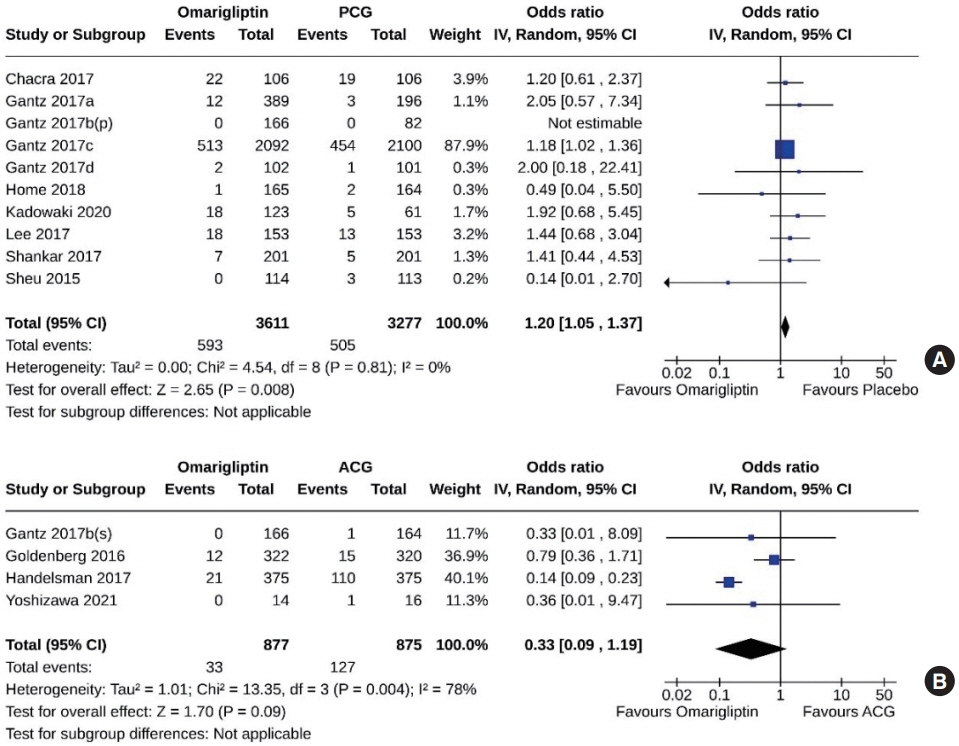
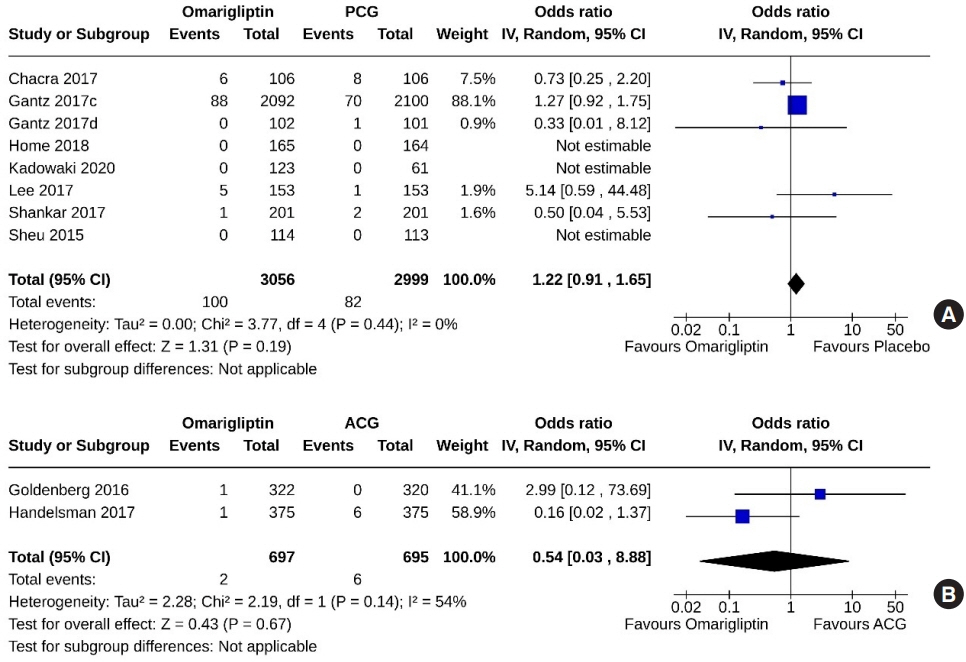
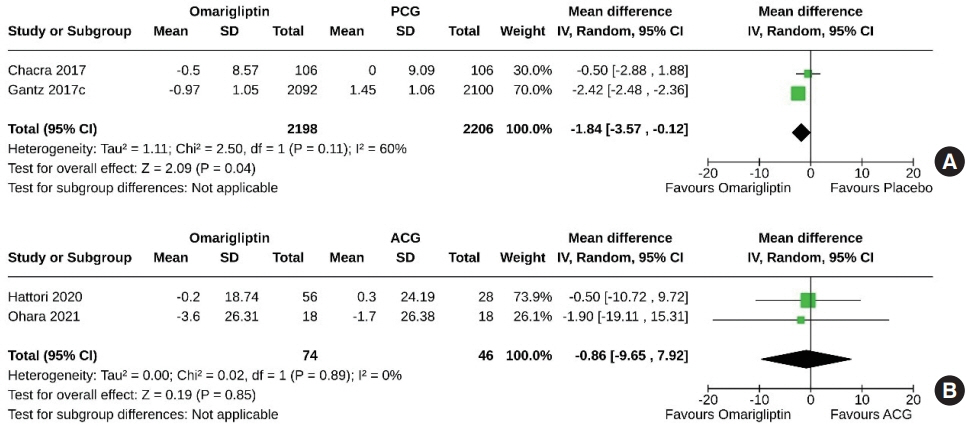
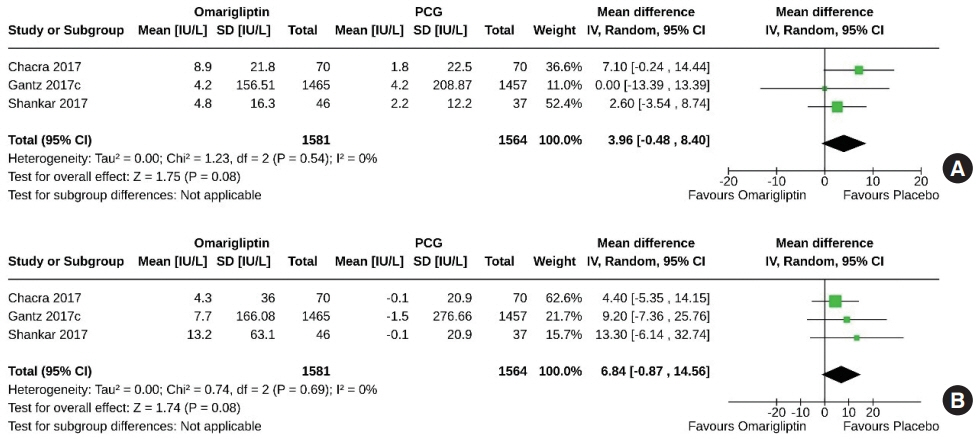
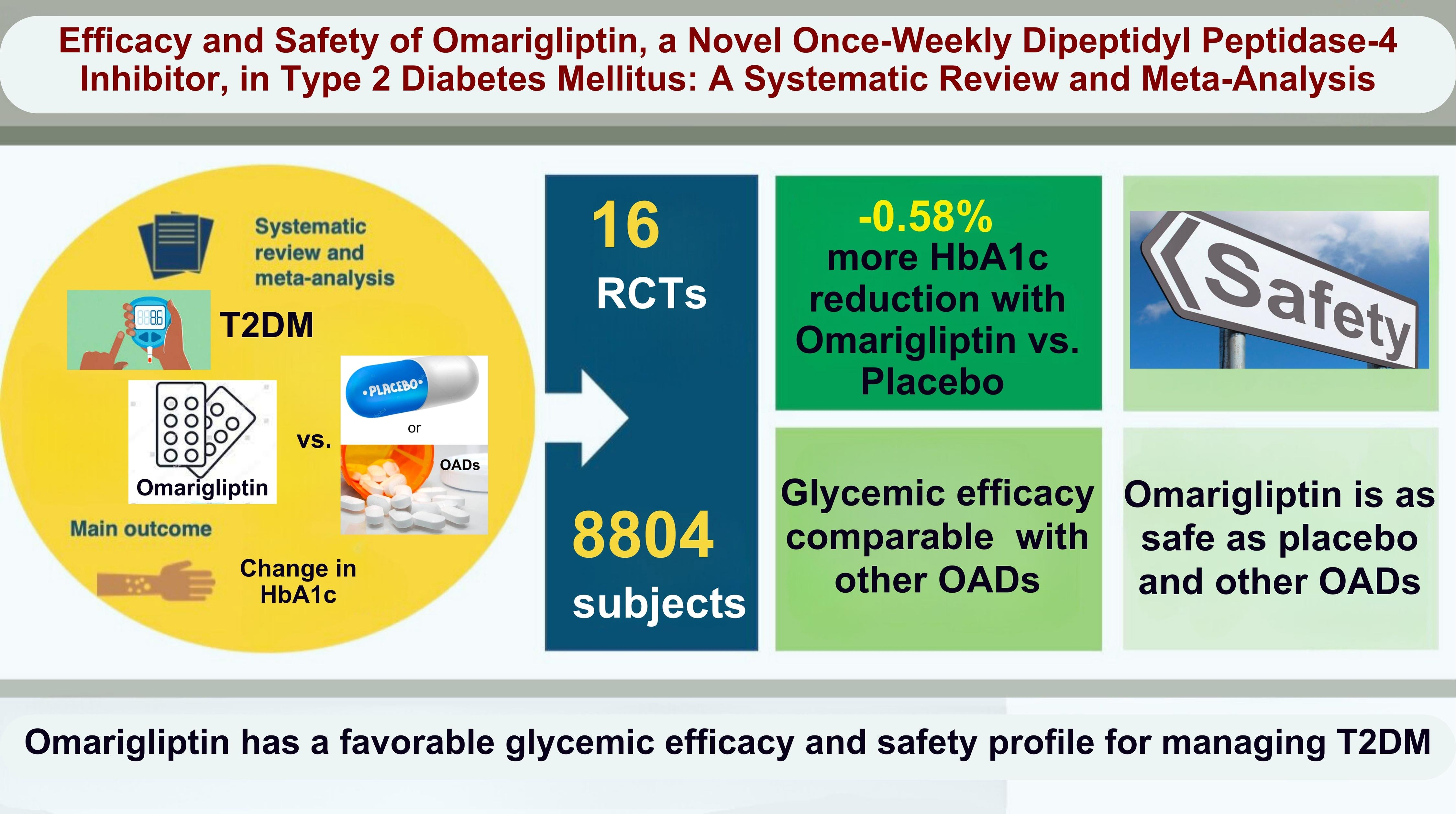
From 332 initially screened articles, data from 16 RCTs involving 8,804 subjects were analyzed. Omarigliptin demonstrated superiority over placebo in reducing HbA1c levels (mean difference, –0.58%; 95% confidence interval, –0.75 to –0.40; P<0.00001; I2=91%). Additionally, omarigliptin outperformed placebo in lowering fasting plasma glucose, 2-hour postprandial glucose, and in the percentage of participants achieving HbA1c levels below 7.0% and 6.5%. The glycemic efficacy of omarigliptin was similar to that of the ACG across all measures. Although the omarigliptin group experienced a higher incidence of hypoglycemic events compared to the PCG, the overall AEs, serious AEs, hypoglycemia, and severe hypoglycemia were comparable between the omarigliptin and control groups (PCG and ACG).
Conclusion
Omarigliptin has a favorable glycemic efficacy and safety profile for managing T2DM.
Keyword
Figure
Reference
-
1. Vella A. Mechanism of action of DPP-4 inhibitors: new insights. J Clin Endocrinol Metab. 2012; 97:2626–8.2. Godinho R, Mega C, Teixeira-de-Lemos E, Carvalho E, Teixeira F, Fernandes R, et al. The place of dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapeutics: a “me too” or “the special one” antidiabetic class? J Diabetes Res. 2015; 2015:806979.3. Burness CB. Omarigliptin: first global approval. Drugs. 2015; 75:1947–52.4. Krishna R, Addy C, Tatosian D, Glasgow XS, Gendrano Iii IN, Robberechts M, et al. Pharmacokinetics and pharmacodynamics of omarigliptin, a once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, after single and multiple doses in healthy subjects. J Clin Pharmacol. 2016; 56:1528–37.5. Tsuchiya S, Friedman E, Addy C, Wakana A, Tatosian D, Matsumoto Y, et al. Single and multiple dose pharmacokinetics and pharmacodynamics of omarigliptin, a novel, once-weekly dipeptidyl peptidase-4 inhibitor, in healthy Japanese men. J Diabetes Investig. 2017; 8:84–92.6. Chacra A, Gantz I, Mendizabal G, Durlach L, O’Neill EA, Zimmer Z, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly DPP-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int J Clin Pract. 2017; 71:e12955.7. Gantz I, Okamoto T, Ito Y, Sato A, Okuyama K, O’Neill EA, et al. A randomized, placebo-controlled trial evaluating the safety and efficacy of adding omarigliptin to antihyperglycemic therapies in Japanese patients with type 2 diabetes and inadequate glycemic control. Diabetes Ther. 2017; 8:793–810.8. Gantz I, Okamoto T, Ito Y, Okuyama K, O’Neill EA, Kaufman KD, et al. A randomized, placebo- and sitagliptin-controlled trial of the safety and efficacy of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in Japanese patients with type 2 diabetes. Diabetes Obes Metab. 2017; 19:1602–9.9. Gantz I, Chen M, Suryawanshi S, Ntabadde C, Shah S, O’Neill EA, et al. A randomized, placebo-controlled study of the cardiovascular safety of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2017; 16:112.10. Gantz I, Sokolova L, Jain L, Iredale C, O’Neill EA, Wei Z, et al. Use of prohibited medication, a potentially overlooked confounder in clinical trials: omarigliptin (once-weekly DPP4 inhibitor) monotherapy trial in 18- to 45-year-olds. Clin Ther. 2017; 39:2024–37.11. Goldenberg R, Gantz I, Andryuk PJ, O’Neill EA, Kaufman KD, Lai E, et al. Randomized clinical trial comparing the efficacy and safety of treatment with the once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor omarigliptin or the oncedaily DPP-4 inhibitor sitagliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Diabetes Obes Metab. 2017; 19:394–400.12. Handelsman Y, Lauring B, Gantz I, Iredale C, O’Neill EA, Wei Z, et al. A randomized, double-blind, non-inferiority trial evaluating the efficacy and safety of omarigliptin, a onceweekly DPP-4 inhibitor, or glimepiride in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin. 2017; 33:1861–8.13. Hattori S. Omarigliptin decreases inflammation and insulin resistance in a pleiotropic manner in patients with type 2 diabetes. Diabetol Metab Syndr. 2020; 12:24.14. Home P, Shankar RR, Gantz I, Iredale C, O’Neill EA, Jain L, et al. A randomized, double-blind trial evaluating the efficacy and safety of monotherapy with the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in people with type 2 diabetes. Diabetes Res Clin Pract. 2018; 138:253–61.15. Ishii H, Kamei N, Shimono D, Niiya T, Tosaki T, Kitazawa T, et al. Treatment burden on once-weekly omarigliptin versus daily dipeptidyl peptidase-4 inhibitors in patients with type 2 diabetes: randomized controlled trial (ONWARD-DPP4 Study). Diabetes Ther. 2023; 14:1639–58.16. Kadowaki T, Seino Y, Kaku K, Okamoto T, Kameya M, Sato A, et al. A randomized, placebo-controlled study to evaluate the efficacy and safety of adding omarigliptin to insulin therapy in Japanese patients with type 2 diabetes and inadequate glycaemic control. Diabetes Obes Metab. 2021; 23:1242–51.17. Lee SH, Gantz I, Round E, Latham M, O’Neill EA, Ceesay P, et al. A randomized, placebo-controlled clinical trial evaluating the safety and efficacy of the once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus inadequately controlled by glimepiride and metformin. BMC Endocr Disord. 2017; 17:70.18. Ohara M, Nagaike H, Fujikawa T, Kohata Y, Ogawa M, Omachi T, et al. Effects of omarigliptin on glucose variability and oxidative stress in type 2 diabetes patients: a prospective study. Diabetes Res Clin Pract. 2021; 179:108999.19. Shankar RR, Inzucchi SE, Scarabello V, Gantz I, Kaufman KD, Lai E, et al. A randomized clinical trial evaluating the efficacy and safety of the once-weekly dipeptidyl peptidase-4 inhibitor omarigliptin in patients with type 2 diabetes inadequately controlled on metformin monotherapy. Curr Med Res Opin. 2017; 33:1853–60.20. Sheu WH, Gantz I, Chen M, Suryawanshi S, Mirza A, Goldstein BJ, et al. Safety and efficacy of omarigliptin (MK-3102), a novel once-weekly DPP-4 inhibitor for the treatment of patients with type 2 diabetes. Diabetes Care. 2015; 38:2106–14.21. Yoshizawa Y, Hosojima M, Kabasawa H, Tanabe N, Ugamura D, Koda Y, et al. Effects of the once-weekly DPP4 inhibitor omarigliptin on glycemic control in patients with type 2 diabetes mellitus on maintenance hemodialysis: a 24-week open-label, multicenter randomized controlled study. Diabetes Ther. 2021; 12:655–67.22. Kawasaki E, Nakano Y, Fukuyama T, Uchida A, Sagara Y, Tamai H, et al. Efficacy of omarigliptin, once-weekly dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes. World J Diabetes. 2021; 12:2087–95.23. Wang X, Li X, Qie S, Zheng Y, Liu Y, Liu G. The efficacy and safety of once-weekly DPP-4 inhibitor omarigliptin in patients with type 2 diabetes mellitus: a systemic review and meta-analysis. Medicine (Baltimore). 2018; 97:e11946.24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71.25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327:557–60.26. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–94.27. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4. (updated August 2023). Chichester: John Wiley & Sons;2023. Chapter 13, Assessing risk of bias due to missing results in a synthesis [cited 2023 Dec 19]. Available from: www.training.cochrane.org/handbook.28. Addy C, Tatosian D, Glasgow XS, Gendrano IN 3rd, Kauh E, Martucci A, et al. Pharmacokinetic and pharmacodynamic effects of multiple-dose administration of omarigliptin, a once-weekly dipeptidyl peptidase-4 inhibitor, in obese participants with and without type 2 diabetes mellitus. Clin Ther. 2016; 38:516–30.29. Addy C, Tatosian DA, Glasgow XS, Gendrano IN III, Sisk CM, Kauh EA, et al. Effects of age, sex, and obesity on the single-dose pharmacokinetics of omarigliptin in healthy subjects. Clin Pharmacol Drug Dev. 2016; 5:374–82.30. Xu S, Tatosian D, Mcintosh I, Caceres M, Matthews C, Samuel K, et al. Absorption, metabolism and excretion of [14C] omarigliptin, a once-weekly DPP-4 inhibitor, in humans. Xenobiotica. 2018; 48:584–91.31. Jain L, Chain AS, Tatosian DA, Hing J, Passarell JA, Kauh EA, et al. Pharmacokinetic-pharmacodynamic (dipeptidyl peptidase-4 inhibition) model to support dose rationale in diabetes patients, including those with renal impairment, for once-weekly administered omarigliptin. Br J Clin Pharmacol. 2019; 85:2759–71.32. Tosaki T, Kamiya H, Yamamoto Y, Himeno T, Kato Y, Kondo M, et al. Efficacy and patient satisfaction of the weekly DPP-4 inhibitors trelagliptin and omarigliptin in 80 Japanese patients with type 2 diabetes. Intern Med. 2017; 56:2563–9.33. Dutta D, Mohindra R, Surana V, Sharma M. Safety and efficacy of once weekly dipeptidyl-peptidase-4 inhibitor trelagliptin in type-2 diabetes: a meta-analysis. Diabetes Metab Syndr. 2022; 16:102469.34. Huang J, Jia Y, Sun S, Meng L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol Toxicol. 2020; 21:68.35. Ling J, Cheng P, Ge L, Zhang DH, Shi AC, Tian JH, et al. The efficacy and safety of dipeptidyl peptidase-4 inhibitors for type 2 diabetes: a Bayesian network meta-analysis of 58 randomized controlled trials. Acta Diabetol. 2019; 56:249–72.36. Florentin M, Kostapanos MS, Papazafiropoulou AK. Role of dipeptidyl peptidase 4 inhibitors in the new era of antidiabetic treatment. World J Diabetes. 2022; 13:85–96.
- Full Text Links
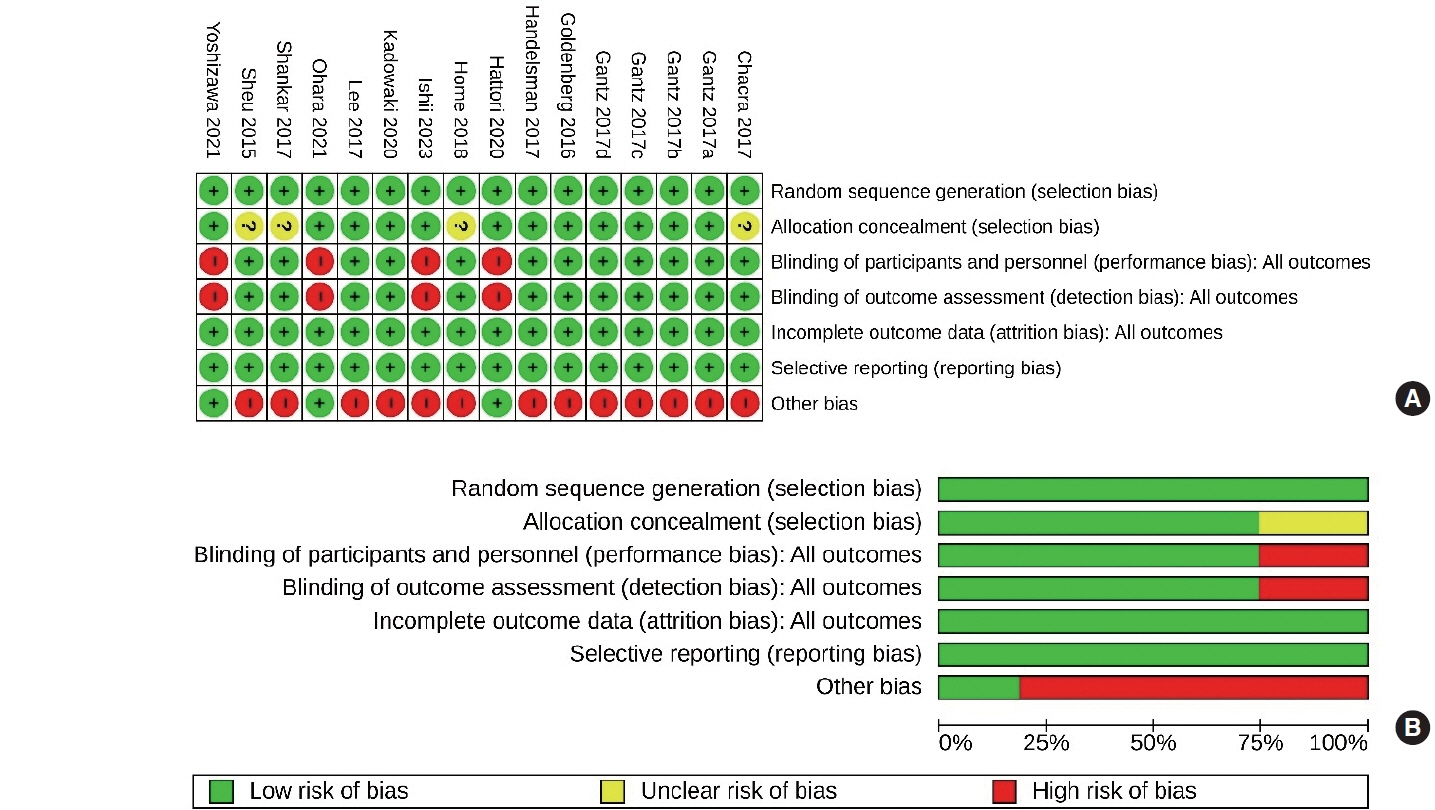
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Novel Therapies for Type 2 Diabetes Mellitus
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
- Dipeptidyl Peptidase-4 Inhibitors and COVID-19-Related Deaths among Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Observational Studies