Endocrinol Metab.
2024 Feb;39(1):47-60. 10.3803/EnM.2024.1937.
Active Surveillance for Low-Risk Thyroid Cancers: A Review of Current Practice Guidelines
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, National Cancer Center, Goyang, Korea
- 4Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul, Korea
- 5Department of Internal Medicine, Nowon Eulji Medical Center, Eulji University, Seoul, Korea
- 6Department of Radiology, Seoul National University Hospital, Seoul, Korea
- 7Deparment of Radiology, Seoul National University College of Medicine, Seoul, Korea
- 8Deparment of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea
- 9Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 10Integrated Major in Innovative Medical Science, Seoul National University College of Medicine, Seoul, Korea
- 11Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Korea
- KMID: 2552795
- DOI: http://doi.org/10.3803/EnM.2024.1937
Abstract
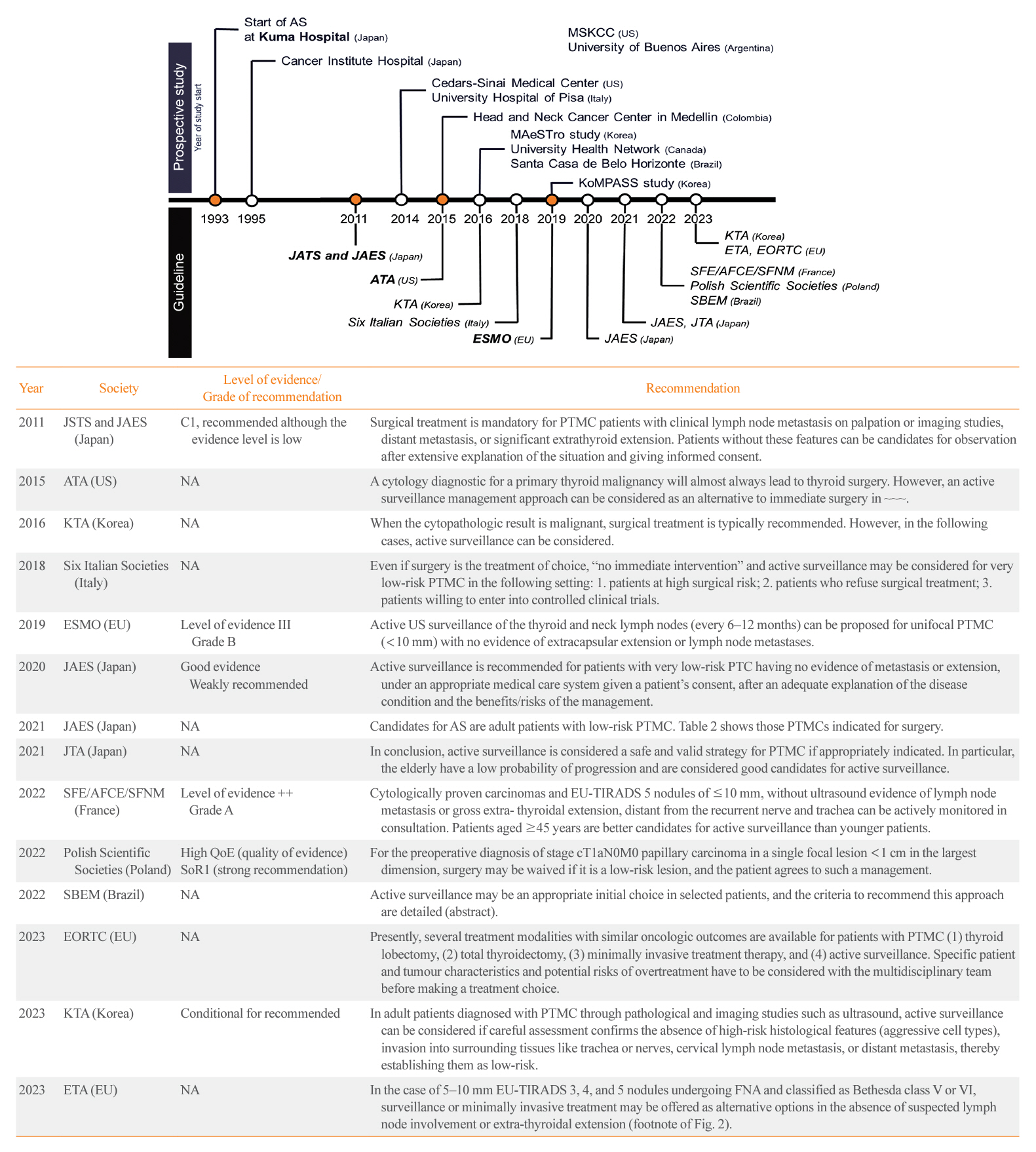
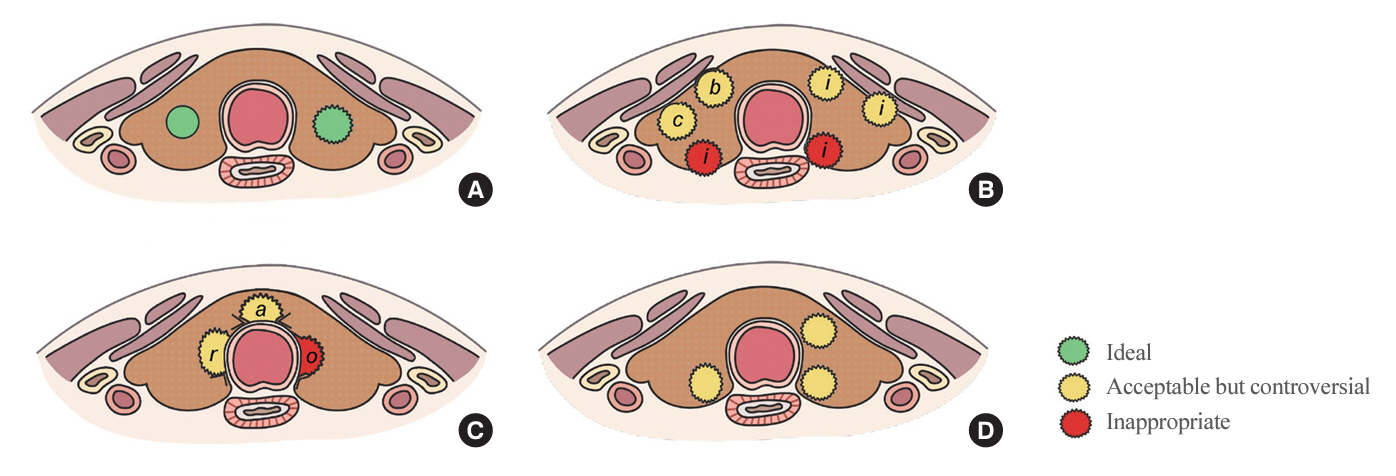
- The indolent nature and favorable outcomes associated with papillary thyroid microcarcinoma have prompted numerous prospective studies on active surveillance (AS) and its adoption as an alternative to immediate surgery in managing low-risk thyroid cancer. This article reviews the current status of AS, as outlined in various international practice guidelines. AS is typically recommended for tumors that measure 1 cm or less in diameter and do not exhibit aggressive subtypes on cytology, extrathyroidal extension, lymph node metastasis, or distant metastasis. To determine the most appropriate candidates for AS, factors such as tumor size, location, multiplicity, and ultrasound findings are considered, along with patient characteristics like medical condition, age, and family history. Moreover, shared decision-making, which includes patient-reported outcomes such as quality of life and cost-effectiveness, is essential. During AS, patients undergo regular ultrasound examinations to monitor for signs of disease progression, including tumor growth, extrathyroidal extension, or lymph node metastasis. In conclusion, while AS is a feasible and reliable approach for managing lowrisk thyroid cancer, it requires careful patient selection, effective communication for shared decision-making, standardized follow-up protocols, and a clear definition of disease progression.
Keyword
Figure
Cited by 1 articles
-
Prediction of Recurrent Laryngeal Nerve Invasion in Thyroid Cancer by Ultrasound
Jin Hyang Jung, Eunji Kim, Byung Ju Kang
J Surg Ultrasound. 2024;11(1):18-24. doi: 10.46268/jsu.2024.11.1.18.
Reference
-
1. Pizzato M, Li M, Vignat J, Laversanne M, Singh D, La Vecchia C, et al. The epidemiological landscape of thyroid cancer worldwide: GLOBOCAN estimates for incidence and mortality rates in 2020. Lancet Diabetes Endocrinol. 2022; 10:264–72.2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017; 317:1338–48.3. Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic?: the increasing impact of overdiagnosis. N Engl J Med. 2016; 375:614–7.4. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”: screening and overdiagnosis. N Engl J Med. 2014; 371:1765–7.5. Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: a “normal” finding in Finland: a systematic autopsy study. Cancer. 1985; 56:531–8.6. Ullmann TM, Papaleontiou M, Sosa JA. Current controversies in low-risk differentiated thyroid cancer: reducing overtreatment in an era of overdiagnosis. J Clin Endocrinol Metab. 2023; 108:271–80.7. Mazzaferri EL. Management of low-risk differentiated thyroid cancer. Endocr Pract. 2007; 13:498–512.8. Hay ID. Management of patients with low-risk papillary thyroid carcinoma. Endocr Pract. 2007; 13:521–33.9. Mehanna H, Al-Maqbili T, Carter B, Martin E, Campain N, Watkinson J, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014; 99:2834–43.10. Tuttle RM, Fagin JA, Minkowitz G, Wong RJ, Roman B, Patel S, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017; 143:1015–20.11. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014; 24:27–34.12. Ito Y, Uruno T, Nakano K, Takamura Y, Miya A, Kobayashi K, et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid. 2003; 13:381–7.13. Takami H, Ito Y, Okamoto T, Yoshida A. Therapeutic strategy for differentiated thyroid carcinoma in Japan based on a newly established guideline managed by Japanese Society of Thyroid Surgeons and Japanese Association of Endocrine Surgeons. World J Surg. 2011; 35:111–21.14. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Papillary microcarcinoma of the thyroid: how should it be treated? World J Surg. 2004; 28:1115–21.15. Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010; 34:28–35.16. Oda H, Miyauchi A, Ito Y, Yoshioka K, Nakayama A, Sasai H, et al. Incidences of unfavorable events in the management of low-risk papillary microcarcinoma of the thyroid by active surveillance versus immediate surgery. Thyroid. 2016; 26:150–5.17. Oda H, Miyauchi A, Ito Y, Sasai H, Masuoka H, Yabuta T, et al. Comparison of the costs of active surveillance and immediate surgery in the management of low-risk papillary microcarcinoma of the thyroid. Endocr J. 2017; 64:59–64.18. Miyauchi A, Kudo T, Ito Y, Oda H, Sasai H, Higashiyama T, et al. Estimation of the lifetime probability of disease progression of papillary microcarcinoma of the thyroid during active surveillance. Surgery. 2018; 163:48–52.19. Miyauchi A, Kudo T, Ito Y, Oda H, Yamamoto M, Sasai H, et al. Natural history of papillary thyroid microcarcinoma: kinetic analyses on tumor volume during active surveillance and before presentation. Surgery. 2019; 165:25–30.20. Miyauchi A, Ito Y, Fujishima M, Miya A, Onoda N, Kihara M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023; 33:817–25.21. Ito Y, Miyauchi A, Fujishima M, Noda T, Sano T, Sasaki T, et al. Thyroid-stimulating hormone, age, and tumor size are risk factors for progression during active surveillance of low-risk papillary thyroid microcarcinoma in adults. World J Surg. 2023; 47:392–401.22. Sasaki T, Miyauchi A, Fujishima M, Ito Y, Kudo T, Noda T, et al. Comparison of postoperative unfavorable events in patients with low-risk papillary thyroid carcinoma: immediate surgery versus conversion surgery following active surveillance. Thyroid. 2023; 33:186–91.23. Yamamoto M, Miyauchi A, Ito Y, Fujishima M, Sasaki T, Kudo T. Active surveillance outcomes of patients with low-risk papillary thyroid microcarcinoma according to levothyroxine treatment status. Thyroid. 2023; 33:1182–9.24. Fujishima M, Miyauchi A, Ito Y, Kudo T, Noda T, Sano T, et al. Active surveillance is an excellent management technique for identifying patients with progressive low-risk papillary thyroid microcarcinoma requiring surgical treatment. Endocr J. 2023; 70:411–8.25. Fukuoka O, Sugitani I, Ebina A, Toda K, Kawabata K, Yamada K. Natural history of asymptomatic papillary thyroid microcarcinoma: time-dependent changes in calcification and vascularity during active surveillance. World J Surg. 2016; 40:529–37.26. Moon JH, Kim JH, Lee EK, Lee KE, Kong SH, Kim YK, et al. Study protocol of multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma (MAeSTro). Endocrinol Metab (Seoul). 2018; 33:278–86.27. Kong SH, Ryu J, Kim MJ, Cho SW, Song YS, Yi KH, et al. Longitudinal assessment of quality of life according to treatment options in low-risk papillary thyroid microcarcinoma patients: active surveillance or immediate surgery (interim analysis of MAeSTro). Thyroid. 2019; 29:1089–96.28. Moon JH, Ryu CH, Cho SW, Choi JY, Chung EJ, Hah JH, et al. Effect of initial treatment choice on 2-year quality of life in patients with low-risk papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2021; 106:724–35.29. Lee EK, Moon JH, Hwangbo Y, Ryu CH, Cho SW, Choi JY, et al. Progression of low-risk papillary thyroid microcarcinoma during active surveillance: interim analysis of a multicenter prospective cohort study of active surveillance on papillary thyroid microcarcinoma in Korea. Thyroid. 2022; 32:1328–36.30. Kim K, Choi JY, Kim SJ, Lee EK, Lee YK, Ryu JS, et al. Active surveillance versus immediate surgery for low-risk papillary thyroid microcarcinoma patients in South Korea: a cost-minimization analysis from the MAeSTro Study. Thyroid. 2022; 32:648–56.31. Hwangbo Y, Choi JY, Lee EK, Ryu CH, Cho SW, Chung EJ, et al. A cross-sectional survey of patient treatment choice in a multicenter prospective cohort study on active surveillance of papillary thyroid microcarcinoma (MAeSTro). Thyroid. 2022; 32:772–80.32. Hwang H, Choi JY, Yu HW, Moon JH, Kim JH, Lee EK, et al. Surgical outcomes in patients with low-risk papillary thyroid microcarcinoma from MAeSTro Study: immediate operation versus delayed operation after active surveillance: a multicenter prospective cohort study. Ann Surg. 2023; 278:e1087–95.33. Lee JY, Kim JH, Kim YK, Lee CY, Lee EK, Moon JH, et al. US predictors of papillary thyroid microcarcinoma progression at active surveillance. Radiology. 2023; 309:e230006.34. Jeon MJ, Kang YE, Moon JH, Lim DJ, Lee CY, Lee YS, et al. Protocol for a Korean multicenter prospective cohort study of active surveillance or surgery (KoMPASS) in papillary thyroid microcarcinoma. Endocrinol Metab (Seoul). 2021; 36:359–64.35. Ho AS, Kim S, Zalt C, Melany ML, Chen IE, Vasquez J, et al. Expanded parameters in active surveillance for low-risk papillary thyroid carcinoma: a nonrandomized controlled trial. JAMA Oncol. 2022; 8:1588–96.36. Sawka AM, Ghai S, Tomlinson G, Rotstein L, Gilbert R, Gullane P, et al. A protocol for a Canadian prospective observational study of decision-making on active surveillance or surgery for low-risk papillary thyroid cancer. BMJ Open. 2018; 8:e020298.37. Sawka AM, Ghai S, Tomlinson G, Baxter NN, Corsten M, Imran SA, et al. A protocol for a Pan-Canadian prospective observational study on active surveillance or surgery for very low risk papillary thyroid cancer. Front Endocrinol (Lausanne). 2021; 12:686996.38. Sawka AM, Ghai S, Rotstein L, Irish JC, Pasternak JD, Gullane PJ, et al. A quantitative analysis examining patients’ choice of active surveillance or surgery for managing low-risk papillary thyroid cancer. Thyroid. 2022; 32:255–62.39. Sanabria A. Experience with active surveillance of thyroid low-risk carcinoma in a developing country. Thyroid. 2020; 30:985–91.40. Rosario PW, Mourao GF, Calsolari MR. Active surveillance in adults with low-risk papillary thyroid microcarcinomas: a prospective study. Horm Metab Res. 2019; 51:703–8.41. Smulever A, Pitoia F. Active surveillance in papillary thyroid carcinoma: not easily accepted but possible in Latin America. Arch Endocrinol Metab. 2019; 63:462–9.42. Molinaro E, Campopiano MC, Pieruzzi L, Matrone A, Agate L, Bottici V, et al. Active surveillance in papillary thyroid microcarcinomas is feasible and safe: experience at a single Italian center. J Clin Endocrinol Metab. 2020; 105:e172–80.43. Campopiano MC, Matrone A, Rago T, Scutari M, Prete A, Agate L, et al. Assessing mPTC progression during active surveillance: volume or diameter increase? J Clin Med. 2021; 10:4068.44. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.45. Yi KH, Lee EK, Kang HC, Koh Y, Kim SW, Kim IJ, et al. 2016 Revised Korean Thyroid Association management guidelines for patients with thyroid nodules and thyroid cancer. Int J Thyroidol. 2016; 9:59–126.46. Pacini F, Basolo F, Bellantone R, Boni G, Cannizzaro MA, De Palma M, et al. Italian consensus on diagnosis and treatment of differentiated thyroid cancer: joint statements of six Italian societies. J Endocrinol Invest. 2018; 41:849–76.47. Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019; 30:1856–83.48. Ito Y, Onoda N, Okamoto T. The revised clinical practice guidelines on the management of thyroid tumors by the Japan Associations of Endocrine Surgeons: core questions and recommendations for treatments of thyroid cancer. Endocr J. 2020; 67:669–717.49. Horiguchi K, Yoshida Y, Iwaku K, Emoto N, Kasahara T, Sato J, et al. Position paper from the Japan Thyroid Association task force on the management of low-risk papillary thyroid microcarcinoma (T1aN0M0) in adults. Endocr J. 2021; 68:763–80.50. Sugitani I, Ito Y, Takeuchi D, Nakayama H, Masaki C, Shindo H, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: consensus statements from the Japan Association of Endocrine Surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021; 31:183–92.51. Leboulleux S, Lamartina L, Lecornet Sokol E, Menegaux F, Leenhardt L, Russ G. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: follow-up: how and how long? Ann Endocrinol (Paris). 2022; 83:407–14.52. Jarzab B, Dedecjus M, Slowinska-Klencka D, Lewinski A, Adamczewski Z, Anielski R, et al. Guidelines of Polish National Societies Diagnostics and Treatment of Thyroid Carcinoma: 2018 update. Endokrynol Pol. 2018; 69:34–74.53. Ward LS, Scheffel RS, Hoff AO, Ferraz C, Vaisman F. Treatment strategies for low-risk papillary thyroid carcinoma: a position statement from the Thyroid Department of the Brazilian Society of Endocrinology and Metabolism (SBEM). Arch Endocrinol Metab. 2022; 66:522–32.54. Koot A, Soares P, Robenshtok E, Locati LD, de la Fouchardiere C, Luster M, et al. Position paper from the endocrine task force of the European Organisation for Research and Treatment of Cancer (EORTC) on the management and shared decision making in patients with low-risk micro papillary thyroid carcinoma. Eur J Cancer. 2023; 179:98–112.55. Park YJ, Lee EK, Song YS, Kang SH, Koo BS, Kim SW, et al. 2023 Korean Thyroid Association management guidelines for patients with thyroid nodules. Int J Thyroidol. 2023; 16:1–31.56. Durante C, Hegedus L, Czarniecka A, Paschke R, Russ G, Schmitt F, et al. 2023 European Thyroid Association clinical practice guidelines for thyroid nodule management. Eur Thyroid J. 2023; 12:e230067.57. Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016; 26:144–9.58. Chou R, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: a systematic review. Thyroid. 2022; 32:351–67.59. Sakai T, Sugitani I, Ebina A, Fukuoka O, Toda K, Mitani H, et al. Active surveillance for T1bN0M0 papillary thyroid carcinoma. Thyroid. 2019; 29:59–63.60. Jarzab B, Dedecjus M, Lewinski A, Adamczewski Z, Bakula-Zalewska E, Baldys-Waligorska A, et al. Diagnosis and treatment of thyroid cancer in adult patients: recommendations of Polish Scientific Societies and the National Oncological Strategy. 2022 Update. Endokrynol Pol. 2022; 73:173–300.61. Ito Y, Miyauchi A, Oda H, Kobayashi K, Kihara M, Miya A. Revisiting low-risk thyroid papillary microcarcinomas resected without observation: was immediate surgery necessary? World J Surg. 2016; 40:523–8.62. Newman SK, Harries V, Wang L, McGill M, Ganly I, Girshman J, et al. Invasion of a recurrent laryngeal nerve from small well-differentiated papillary thyroid cancers: patient selection implications for active surveillance. Thyroid. 2022; 32:164–9.63. Oh HS, Kwon H, Song E, Jeon MJ, Kim TY, Lee JH, et al. Tumor volume doubling time in active surveillance of papillary thyroid carcinoma. Thyroid. 2019; 29:642–9.64. Yabuta T, Matsuse M, Hirokawa M, Yamashita S, Mitsutake N, Miyauchi A. TERT promoter mutations were not found in papillary thyroid microcarcinomas that showed disease progression on active surveillance. Thyroid. 2017; 27:1206–7.65. Chen Y, Sadow PM, Suh H, Lee KE, Choi JY, Suh YJ, et al. BRAF(V600E) is correlated with recurrence of papillary thyroid microcarcinoma: a systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid. 2016; 26:248–55.66. Li F, Chen G, Sheng C, Gusdon AM, Huang Y, Lv Z, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer. 2015; 22:159–68.67. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014; 32:2718–26.68. Moon S, Song YS, Kim YA, Lim JA, Cho SW, Moon JH, et al. Effects of coexistent BRAFV600E and TERT promoter mutations on poor clinical outcomes in papillary thyroid cancer: a meta-analysis. Thyroid. 2017; 27:651–60.69. Kim MJ, Kim JK, Kim GJ, Kang SW, Lee J, Jeong JJ, et al. TERT promoter and BRAF V600E mutations in papillary thyroid cancer: a single-institution experience in Korea. Cancers (Basel). 2022; 14:4928.70. Yang H, Park H, Ryu HJ, Heo J, Kim JS, Oh YL, et al. Frequency of TERT promoter mutations in real-world analysis of 2,092 thyroid carcinoma patients. Endocrinol Metab (Seoul). 2022; 37:652–63.71. Uchino S, Noguchi S, Kawamoto H, Yamashita H, Watanabe S, Yamashita H, et al. Familial nonmedullary thyroid carcinoma characterized by multifocality and a high recurrence rate in a large study population. World J Surg. 2002; 26:897–902.72. Capezzone M, Secchi C, Fralassi N, Cantara S, Brilli L, Ciuoli C, et al. Should familial disease be considered as a negative prognostic factor in micropapillary thyroid carcinoma? J Endocrinol Invest. 2019; 42:1205–13.73. Ito Y, Kakudo K, Hirokawa M, Fukushima M, Yabuta T, Tomoda C, et al. Biological behavior and prognosis of familial papillary thyroid carcinoma. Surgery. 2009; 145:100–5.74. Kung AW, Chau MT, Lao TT, Tam SC, Low LC. The effect of pregnancy on thyroid nodule formation. J Clin Endocrinol Metab. 2002; 87:1010–4.75. Ito Y, Miyauchi A, Kudo T, Ota H, Yoshioka K, Oda H, et al. Effects of pregnancy on papillary microcarcinomas of the thyroid re-evaluated in the entire patient series at Kuma Hospital. Thyroid. 2016; 26:156–60.76. Cao Y, Wang Z, Gu J, Hu F, Qi Y, Yin Q, et al. Reproductive factors but not hormonal factors associated with thyroid cancer risk: a systematic review and meta-analysis. Biomed Res Int. 2015; 2015:103515.77. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young age and male sex are predictors of large-volume central neck lymph node metastasis in clinical N0 papillary thyroid microcarcinomas. Thyroid. 2017; 27:1285–90.78. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016; 26:807–15.79. Jeon MJ, Lee YM, Sung TY, Han M, Shin YW, Kim WG, et al. Quality of life in patients with papillary thyroid microcarcinoma managed by active surveillance or lobectomy: a cross-sectional study. Thyroid. 2019; 29:956–62.80. Liu C, Zhao H, Xia Y, Cao Y, Zhang L, Zhao Y, et al. Active surveillance versus immediate surgery: a comparison of clinical and quality of life outcomes among patients with highly suspicious thyroid nodules 1 cm or smaller in China. Eur J Surg Oncol. 2023; 49:106917.81. Yoshida Y, Horiuchi K, Okamoto T. Patients’ view on the management of papillary thyroid microcarcinoma: active surveillance or surgery. Thyroid. 2020; 30:681–7.82. Lang BH, Wong CK. A cost-effectiveness comparison between early surgery and non-surgical approach for incidental papillary thyroid microcarcinoma. Eur J Endocrinol. 2015; 173:367–75.83. Lin JF, Jonker PK, Cunich M, Sidhu SB, Delbridge LW, Glover AR, et al. Surgery alone for papillary thyroid microcarcinoma is less costly and more effective than long term active surveillance. Surgery. 2020; 167:110–6.84. Venkatesh S, Pasternak JD, Beninato T, Drake FT, Kluijfhout WP, Liu C, et al. Cost-effectiveness of active surveillance versus hemithyroidectomy for micropapillary thyroid cancer. Surgery. 2017; 161:116–26.85. Kim HI, Jang HW, Ahn HS, Ahn S, Park SY, Oh YL, et al. High serum TSH level is associated with progression of papillary thyroid microcarcinoma during active surveillance. J Clin Endocrinol Metab. 2018; 103:446–51.86. Sugitani I, Fujimoto Y, Yamada K. Association between serum thyrotropin concentration and growth of asymptomatic papillary thyroid microcarcinoma. World J Surg. 2014; 38:673–8.87. Ito Y, Miyauchi A. A therapeutic strategy for incidentally detected papillary microcarcinoma of the thyroid. Nat Clin Pract Endocrinol Metab. 2007; 3:240–8.88. Park CS, Kim SH, Jung SL, Kang BJ, Kim JY, Choi JJ, et al. Observer variability in the sonographic evaluation of thyroid nodules. J Clin Ultrasound. 2010; 38:287–93.89. Ito Y, Miyauchi A, Kudo T, Higashiyama T, Masuoka H, Kihara M, et al. Kinetic analysis of growth activity in enlarging papillary thyroid microcarcinomas. Thyroid. 2019; 29:1765–73.90. Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European Thyroid Association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017; 6:225–37.91. Loncar I, van Dijk SP, Metman MJ, Lin JF, Kruijff S, Peeters RP, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic workup strategies. Thyroid. 2021; 31:1219–25.92. Do Cao C, Haissaguerre M, Lussey-Lepoutre C, Donatini G, Raverot V, Russ G. SFE-AFCE-SFMN 2022 Consensus on the management of thyroid nodules: initial work-up for thyroid nodules. Ann Endocrinol (Paris). 2022; 83:380–8.93. Zhuge L, Huang Z, Cai H, Wang S, Niu L, Li Z. The optimal age threshold for stratifying the risks of disease progression in patients with highly suspicious sub-centimeter thyroid nodules. Ann Surg Oncol. 2023; 30:5463–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and treatment of low-risk papillary thyroid microcarcinoma
- A Review of Active Surveillance of Papillary Thyroid Microcarcinoma
- Management of Low-Risk Papillary Thyroid Cancer
- Risk Factors for Tumor Size Increase During Active Surveillance of Papillary Thyroid Cancer: Meta-Analysis and Systematic Review
- Active Surveillance of Papillary Thyroid Cancer: Past, Present, and Future