Endocrinol Metab.
2024 Feb;39(1):33-39. 10.3803/EnM.2024.1911.
Glucagon: Physiological and Pharmacological Functions and Pathophysiological Significance in Type 2 Diabetes
- Affiliations
-
- 1Metabolic Signal Research Center, Institute for Molecular and Cellular Regulation, Gunma University, Maebashi, Japan
- KMID: 2552793
- DOI: http://doi.org/10.3803/EnM.2024.1911
Abstract
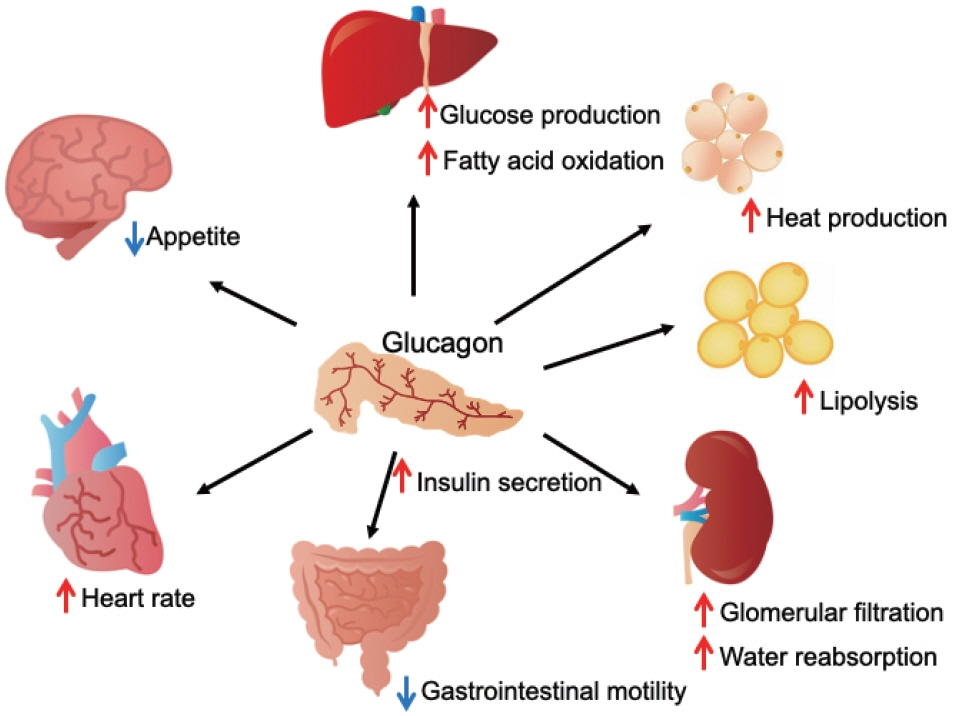
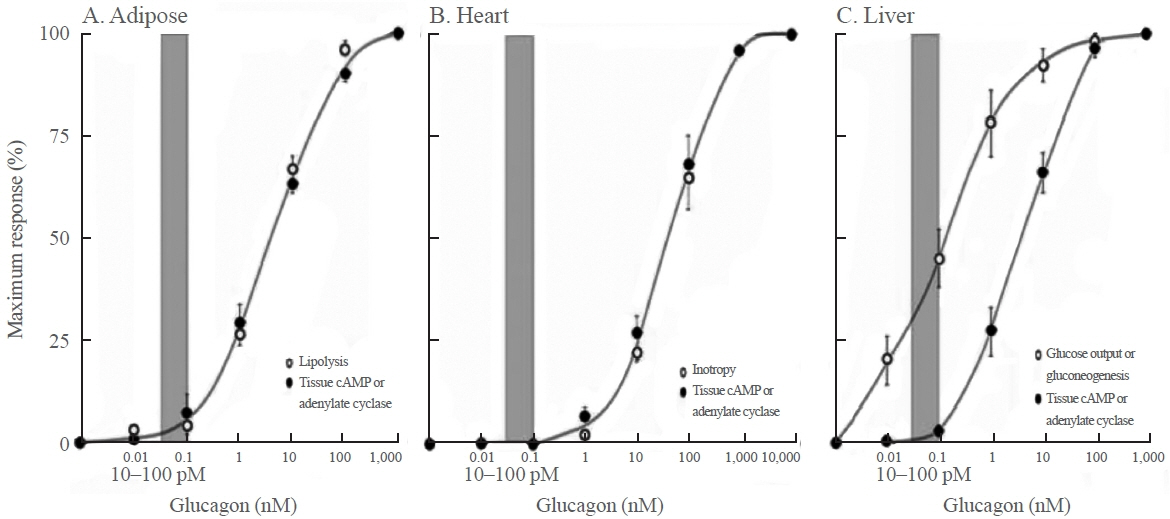
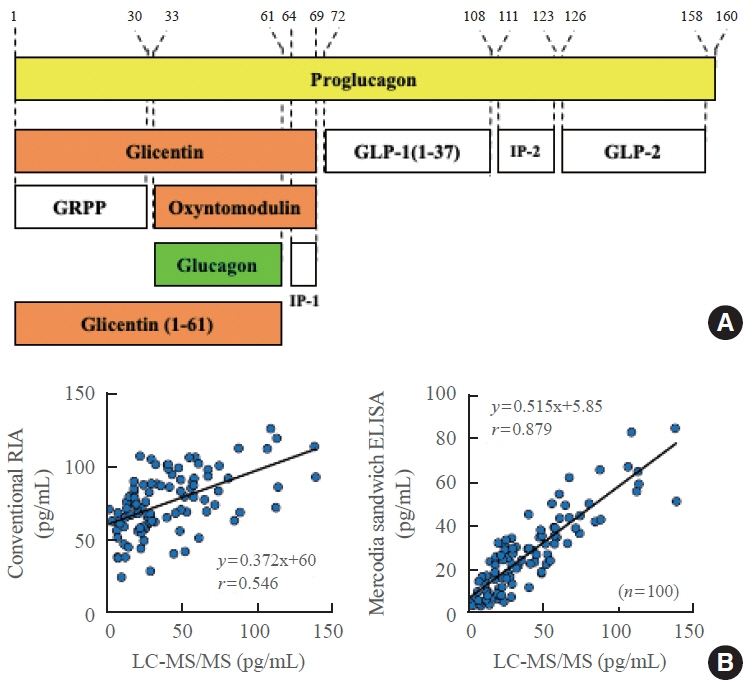
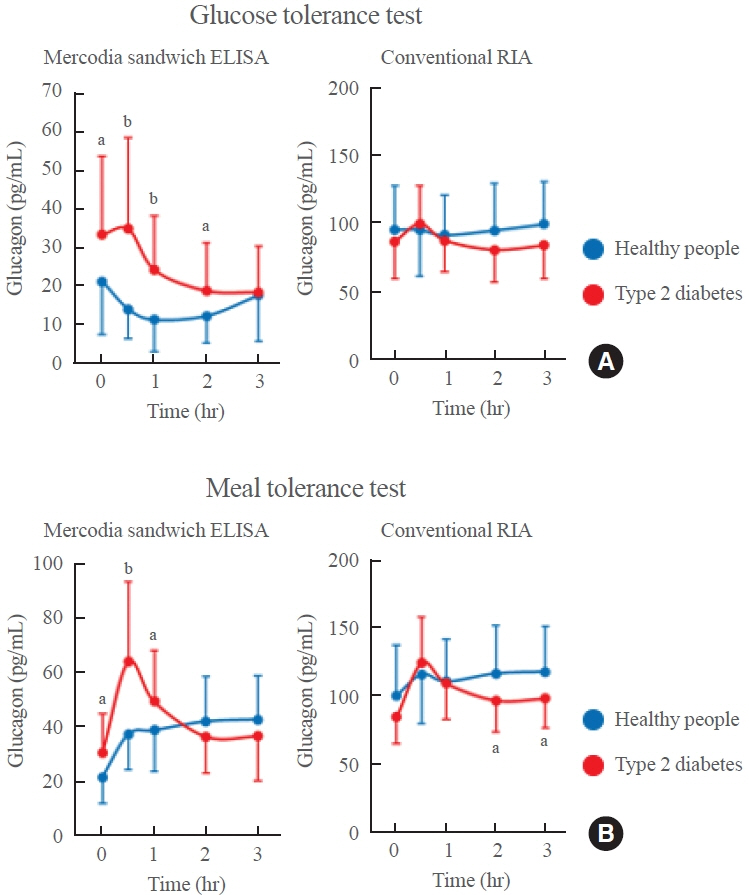
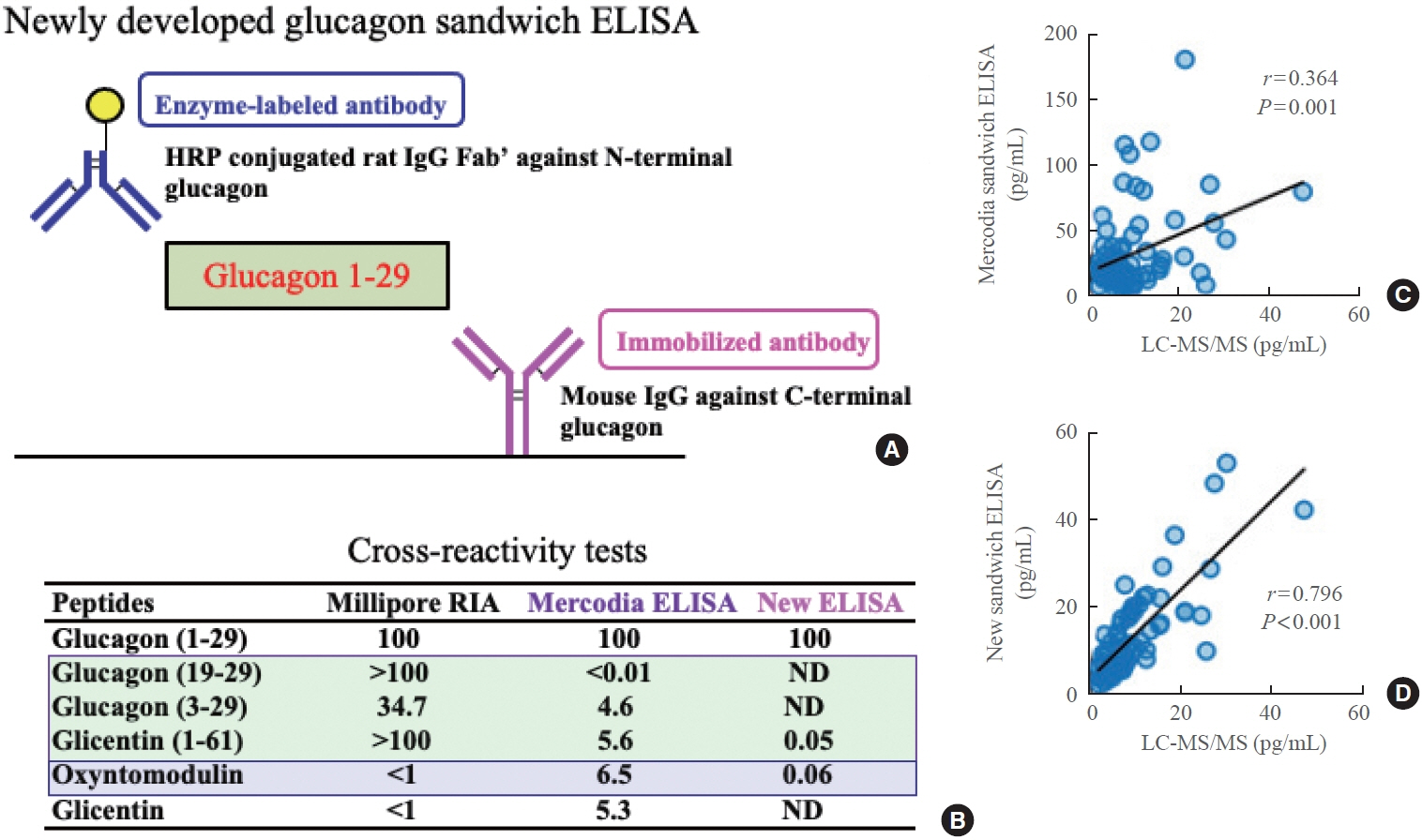
- Glucagon has many functions, including the promotion of hepatic glucose production, fatty acid oxidation, thermogenesis, energy consumption, lipolysis, and myocardial contraction, as well as the suppression of lipogenesis, appetite, and gastrointestinal motility. However, it remains unclear which of these functions are physiological and which are pharmacological. Research on glucagon has lagged behind research on insulin because cross-reactivity with glucagon-related peptides in plasma has hindered the development of an accurate measurement system for glucagon. We recently developed a new glucagon sandwich enzyme-linked immunosorbent assay (ELISA) that is more specific and more sensitive to glucagon than the currently used measurement systems. The new sandwich ELISA is expected to contribute to personalized medicine for diabetes through its use in clinical examinations, the diagnosis of the pathophysiological condition of individual diabetes patients, and the choice of a treatment strategy. Efforts are continuing to develop glucagon/glucagon-like peptide-1 receptor dual agonists to improve obesity and fatty liver by enhancing glucagon’s appetite-suppressing and lipolysis- and thermogenesis-promoting effects. Thus, glucagon is expected to be applied to new diagnostic and therapeutic strategies based on a more accurate understanding of its functions.
Figure
Reference
-
1. Rodgers RL. Glucagon and cyclic AMP: time to turn the page? Curr Diabetes Rev. 2012; 8:362–81.2. Bak MJ, Albrechtsen NW, Pedersen J, Hartmann B, Christensen M, Vilsboll T, et al. Specificity and sensitivity of commercially available assays for glucagon and oxyntomodulin measurement in humans. Eur J Endocrinol. 2014; 170:529–38.3. Wewer Albrechtsen NJ, Hartmann B, Veedfald S, Windelov JA, Plamboeck A, Bojsen-Moller KN, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014; 57:1919–26.4. Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive hyperglucagonemia in patients with type 2 diabetes mellitus analyzed with a novel enzymelinked immunosorbent assay. J Diabetes Investig. 2016; 7:324–31.5. Miyachi A, Kobayashi M, Mieno E, Goto M, Furusawa K, Inagaki T, et al. Accurate analytical method for human plasma glucagon levels using liquid chromatography-high resolution mass spectrometry: comparison with commercially available immunoassays. Anal Bioanal Chem. 2017; 409:5911–8.6. Kobayashi M, Satoh H, Matsuo T, Kusunoki Y, Tokushima M, Watada H, et al. Plasma glucagon levels measured by sandwich ELISA are correlated with impaired glucose tolerance in type 2 diabetes. Endocr J. 2020; 67:903–22.7. Kobayashi M, Waki H, Nakayama H, Miyachi A, Mieno E, Hamajima H, et al. Pseudo-hyperglucagonemia was observed in pancreatectomized patients when measured by glucagon sandwich enzyme-linked immunosorbent assay. J Diabetes Investig. 2021; 12:286–9.8. Kobayashi M, Maruyama N, Yamamoto Y, Togawa T, Ida T, Yoshida M, et al. A newly developed glucagon sandwich ELISA is useful for more accurate glucagon evaluation than the currently used sandwich ELISA in subjects with elevated plasma proglucagon-derived peptide levels. J Diabetes Investig. 2023; 14:648–58.9. Suga T, Kikuchi O, Kobayashi M, Matsui S, Yokota-Hashimoto H, Wada E, et al. SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol Metab. 2019; 19:1–12.10. Day JW, Ottaway N, Patterson JT, Gelfanov V, Smiley D, Gidda J, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol. 2009; 5:749–57.11. Ambery P, Parker VE, Stumvoll M, Posch MG, Heise T, Plum-Moerschel L, et al. MEDI0382, a GLP-1 and glucagon receptor dual agonist, in obese or overweight patients with type 2 diabetes: a randomised, controlled, double-blind, ascending dose and phase 2a study. Lancet. 2018; 391:2607–18.12. Alba M, Yee J, Frustaci ME, Samtani MN, Fleck P. Efficacy and safety of glucagon-like peptide-1/glucagon receptor coagonist JNJ-64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose-ranging study. Clin Obes. 2021; 11:e12432.13. Romero-Gomez M, Lawitz E, Shankar RR, Chaudhri E, Liu J, Lam RLH, et al. A phase IIa active-comparator-controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non-alcoholic fatty liver disease. J Hepatol. 2023; 79:888–97.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incretin Hormones: Pathophysiological Risk Factors and Potential Targets for Type 2 Diabetes
- Effects of Sitagliptin on Insulin and Glucagon Levels in Type 2 Diabetes Mellitus
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- Clinical Efficacy of Glucagon Like Peptide-1 (GLP-1) Analogues