Korean J Physiol Pharmacol.
2024 Jan;28(1):11-19. 10.4196/kjpp.2024.28.1.11.
Aurantio-obtusin exerts an anti-inflammatory effect on acute kidney injury by inhibiting NF-κκB pathway
- Affiliations
-
- 1Department of Nephrology, Wuhan Sixth Hospital, Affiliated Hospital of Jianghan University, Wuhan 430014, Hubei, China
- KMID: 2550275
- DOI: http://doi.org/10.4196/kjpp.2024.28.1.11
Abstract
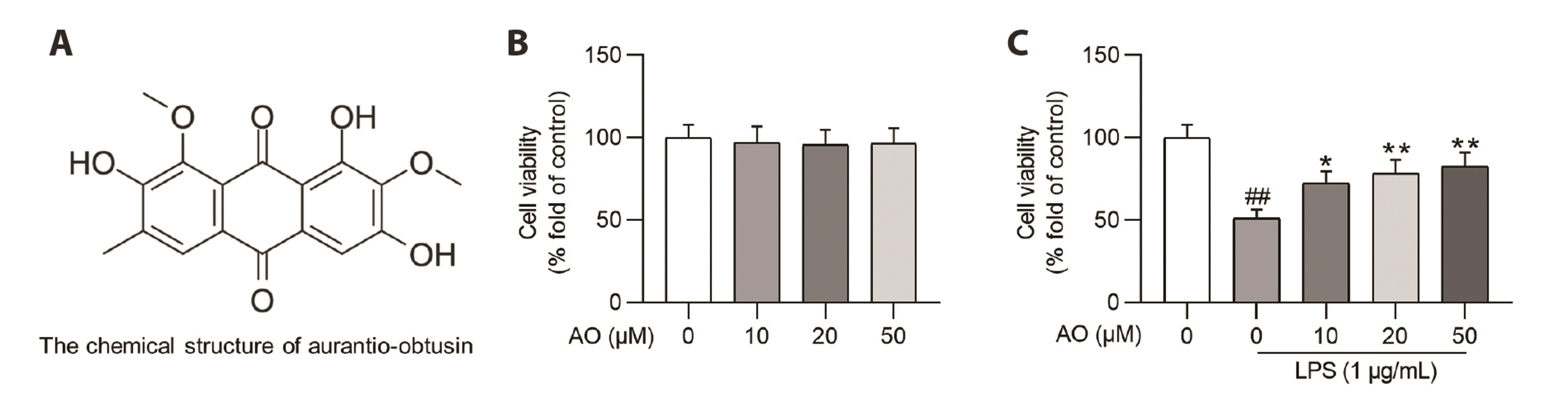
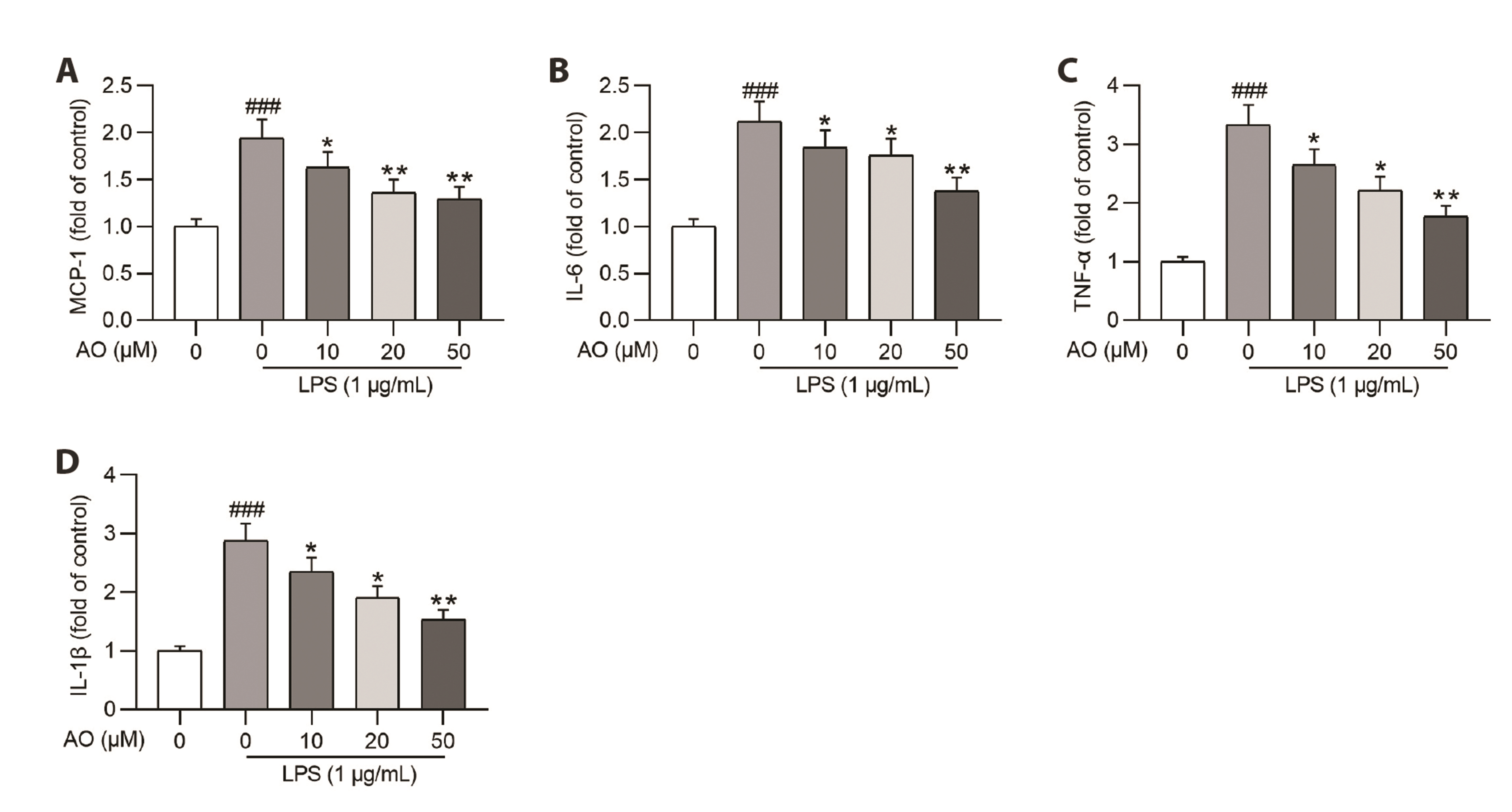
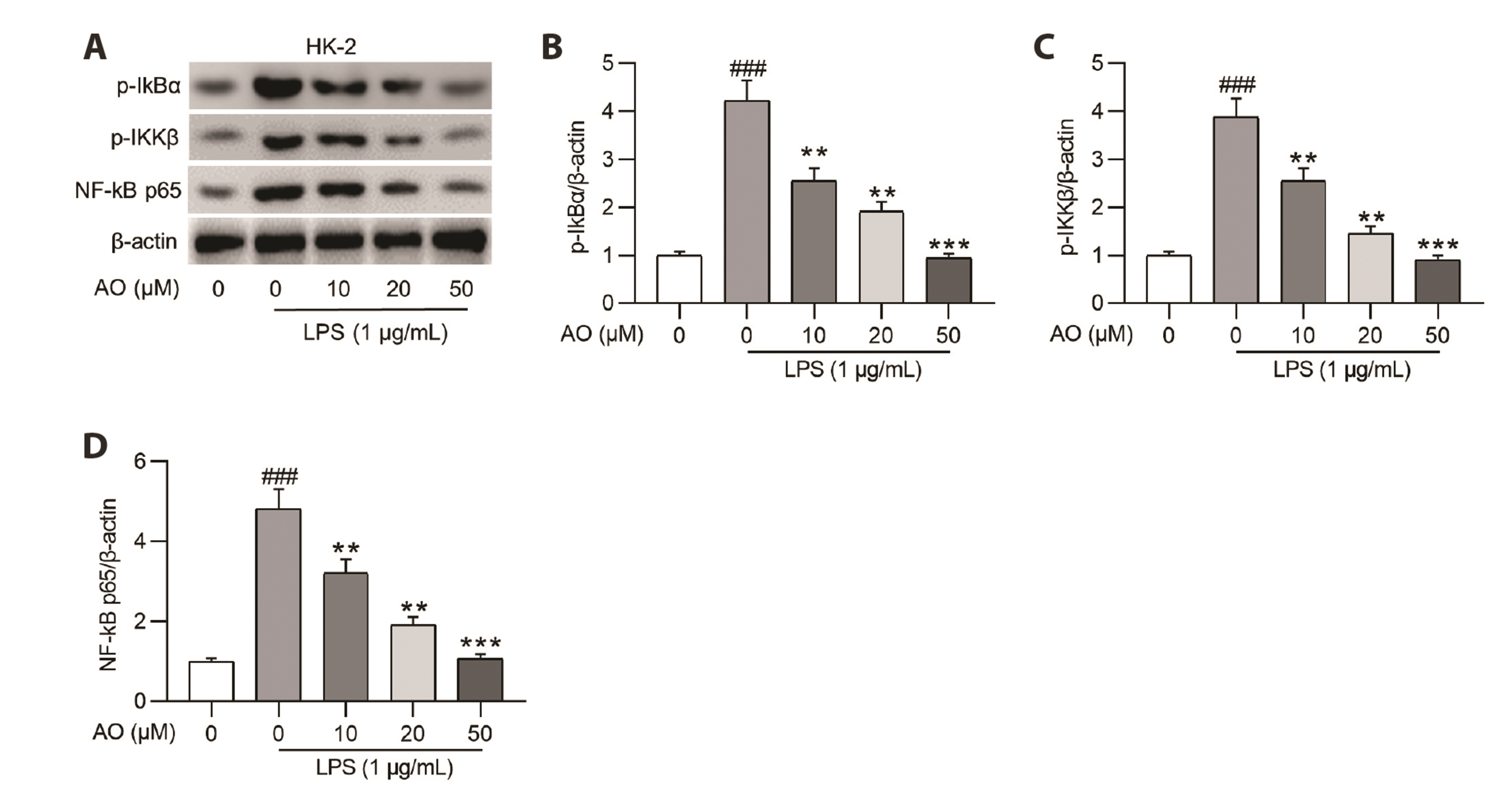
- Acute kidney injury (AKI) is one of the major complications of sepsis. Aurantio-obtusin (AO) is an anthraquinone compound with antioxidant and antiinflammatory activities. This study was developed to concentrate on the role and mechanism of AO in sepsis-induced AKI. Lipopolysaccharide (LPS)-stimulated human renal proximal tubular epithelial cells (HK-2) and BALB/c mice receiving cecal ligation and puncture (CLP) surgery were used to establish in vitro cell model and in vivo mouse model. HK-2 cell viability was measured using MTT assays. Histological alterations of mouse renal tissues were analyzed via hematoxylin and eosin staining. Renal function of mice was assessed by measuring the levels of serum creatinine (SCr) and blood urea nitrogen (BUN). The concentrations of pro-inflammatory cytokines in HK-2 cells and serum samples of mice were detected using corresponding ELISA kits. Protein levels of factors associated with nuclear factor kappa-B (NF-κB) pathway were measured in HK-2 cells and renal tissues by Western blotting. AO exerted no cytotoxic effect on HK-2 cells and AO dose-dependently rescued LPS-induced decrease in HK-2 cell viability. The concentrations of pro-inflammatory cytokines were increased in response to LPS or CLP treatment, and the alterations were reversed by AO treatment. For in vivo experiments, AO markedly ameliorated renal injury and reduced high levels of SCr and BUN in mice underwent CLP operation. In addition, AO administration inhibited the activation of NF-κB signaling pathway in vitro and in vivo. In conclusion, AO alleviates septic AKI by suppressing inflammatory responses through inhibiting the NF-κB pathway.
Figure
Reference
-
1. Gómez H, Kellum JA. 2016; Sepsis-induced acute kidney injury. Curr Opin Crit Care. 22:546–553. DOI: 10.1097/MCC.0000000000000356. PMID: 27661757. PMCID: PMC5654474.2. Yoon SY, Kim JS, Jeong KH, Kim SK. 2022; Acute kidney injury: biomarker-guided diagnosis and management. Medicina (Kaunas). 58:340. DOI: 10.3390/medicina58030340. PMID: 35334515. PMCID: PMC8953384. PMID: 6bdc694706ab4bad90a0abaff05bd722.3. Manrique-Caballero CL, Del Rio-Pertuz G, Gomez H. 2021; Sepsis-associated acute kidney injury. Crit Care Clin. 37:279–301. DOI: 10.1016/j.ccc.2020.11.010. PMID: 33752856. PMCID: PMC7995616.4. Basile DP, Anderson MD, Sutton TA. 2012; Pathophysiology of acute kidney injury. Compr Physiol. 2:1303–1353. DOI: 10.1002/cphy.c110041. PMID: 23798302. PMCID: PMC3919808.5. Levey AS, James MT. 2017; Acute kidney injury. Ann Intern Med. 167:ITC66–ITC80. Erratum in: Ann Intern Med. 2018;168:84. DOI: 10.7326/AITC201711070. PMID: 29114754.6. Guo J, Wang R, Min F. 2022; Ginsenoside Rg1 ameliorates sepsis-induced acute kidney injury by inhibiting ferroptosis in renal tubular epithelial cells. J Leukoc Biol. 112:1065–1077. DOI: 10.1002/JLB.1A0422-211R. PMID: 35774015.7. Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, Hunn BH. 2017; AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol. 28:377–387. DOI: 10.1681/ASN.2016010105. PMID: 27297949. PMCID: PMC5198285.8. Acedillo RR, Wald R, McArthur E, Nash DM, Silver SA, James MT, Schull MJ, Siew ED, Matheny ME, House AA, Garg AX. 2017; Characteristics and outcomes of patients discharged home from an emergency department with AKI. Clin J Am Soc Nephrol. 12:1215–1225. DOI: 10.2215/CJN.10431016. PMID: 28729384. PMCID: PMC5544515.9. Bindroo S, Quintanilla Rodriguez BS, Challa HJ. 2023. Renal failure. StatPearls Publishing;DOI: 10.1080/0886022x.2023.2241913.10. Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, Yang G, Chen Y, Cheng J, Lu Y, Liu J. 2021; Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 11:1845–1863. DOI: 10.7150/thno.50905. PMID: 33408785. PMCID: PMC7778599.11. Chen Y, Wei W, Fu J, Zhang T, Zhao J, Ma T. 2023; Forsythiaside A ameliorates sepsis-induced acute kidney injury via anti-inflammation and antiapoptotic effects by regulating endoplasmic reticulum stress. BMC Complement Med Ther. 23:35. DOI: 10.1186/s12906-023-03855-7. PMID: 36737765. PMCID: PMC9896724. PMID: 640aad30fc33423586a3d269f2839abf.12. Ding Y, Zheng Y, Huang J, Peng W, Chen X, Kang X, Zeng Q. 2019; UCP2 ameliorates mitochondrial dysfunction, inflammation, and oxidative stress in lipopolysaccharide-induced acute kidney injury. Int Immunopharmacol. 71:336–349. DOI: 10.1016/j.intimp.2019.03.043. PMID: 30952098.13. Lin G, Li N, Li D, Chen L, Deng H, Wang S, Tang J, Ouyang W. 2023; Carnosic acid inhibits NLRP3 inflammasome activation by targeting both priming and assembly steps. Int Immunopharmacol. 116:109819. DOI: 10.1016/j.intimp.2023.109819. PMID: 36738671.14. Ye HY, Jin J, Jin LW, Chen Y, Zhou ZH, Li ZY. 2017; Chlorogenic acid attenuates lipopolysaccharide-induced acute kidney injury by inhibiting TLR4/NF-κB signal pathway. Inflammation. 40:523–529. DOI: 10.1007/s10753-016-0498-9. PMID: 28028753.15. Hu X, Zhou W, Wu S, Wang R, Luan Z, Geng X, Xu N, Zhang Z, Ruan Z, Wang Z, Li F, Yu C, Ren H. 2022; Tacrolimus alleviates LPS-induced AKI by inhibiting TLR4/MyD88/NF-κB signalling in mice. J Cell Mol Med. 26:507–514. DOI: 10.1111/jcmm.17108. PMID: 34889045. PMCID: PMC8743665.16. Lin YY, Tsai SJ, Chiang MY, Wen ZH, Su JH. 2015; Anti-inflammatory anthraquinones from the crinoid Himerometra magnipinna. Nat Prod Commun. 10:317–318. DOI: 10.1177/1934578X1501000227. PMID: 25920272.17. Yang X, Kang MC, Li Y, Kim EA, Kang SM, Jeon YJ. 2014; Anti-inflammatory activity of questinol isolated from marine-derived fungus Eurotium amstelodami in lipopolysaccharide-stimulated RAW 264.7 macrophages. J Microbiol Biotechnol. 24:1346–1353. DOI: 10.4014/jmb.1405.05035. PMID: 24986678.18. Zhang Y, Pu W, Bousquenaud M, Cattin S, Zaric J, Sun LK, Rüegg C. 2021; Emodin inhibits inflammation, carcinogenesis, and cancer progression in the AOM/DSS model of colitis-associated intestinal tumorigenesis. Front Oncol. 10:564674. DOI: 10.3389/fonc.2020.564674. PMID: 33489875. PMCID: PMC7821392. PMID: 2fd1e3fe8d4a4eb6b61ad297a4f7e7a2.19. Hou J, Gu Y, Zhao S, Huo M, Wang S, Zhang Y, Qiao Y, Li X. 2018; Anti-Inflammatory effects of aurantio-obtusin from seed of Cassia obtusifolia L. through modulation of the NF-κB pathway. Molecules. 23:3093. Erratum in: Molecules. 2019;24:745. DOI: 10.3390/molecules24040745. PMID: 30791435. PMCID: PMC6412286. PMID: 27a4ca2c2f704da8bf5a1bb55719b166.20. Kim M, Lim SJ, Lee HJ, Nho CW. 2015; Cassia tora seed extract and its active compound aurantio-obtusin inhibit allergic responses in IgE-mediated mast cells and anaphylactic models. J Agric Food Chem. 63:9037–9046. DOI: 10.1021/acs.jafc.5b03836. PMID: 26434611.21. Ju MS, Kim HG, Choi JG, Ryu JH, Hur J, Kim YJ, Oh MS. 2010; Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson's disease models. Food Chem Toxicol. 48:2037–2044. DOI: 10.1016/j.fct.2010.05.002. PMID: 20457209.22. Kitanaka S, Nakayama T, Shibano T, Ohkoshi E, Takido M. 1998; Antiallergic agent from natural sources. Structures and inhibitory effect of histamine release of naphthopyrone glycosides from seeds of Cassia obtusifolia L. Chem Pharm Bull (Tokyo). 46:1650–1652. DOI: 10.1248/cpb.46.1650. PMID: 9810700.23. Li S, Li Q, Lv X, Liao L, Yang W, Li S, Lu P, Zhu D. 2015; Aurantio-obtusin relaxes systemic arteries through endothelial PI3K/AKT/eNOS-dependent signaling pathway in rats. J Pharmacol Sci. 128:108–115. DOI: 10.1016/j.jphs.2015.05.006. PMID: 26076958. PMID: a36460f5593342b78f5e12b76de8a1f0.24. Kwon KS, Lee JH, So KS, Park BK, Lim H, Choi JS, Kim HP. 2018; Aurantio-obtusin, an anthraquinone from cassiae semen, ameliorates lung inflammatory responses. Phytother Res. 32:1537–1545. DOI: 10.1002/ptr.6082. PMID: 29675883.25. Xiao SL, Guan LJ, Jiang RF, Wang XG, Li X, Cai W. 2020; The metabolism and pharmacokinetics of rhein and aurantio-obtusin. Curr Drug Metab. 21:960–968. DOI: 10.2174/1389200221666200719002128. PMID: 32682364.26. Yu C, Qi D, Sun JF, Li P, Fan HY. 2015; Rhein prevents endotoxin-induced acute kidney injury by inhibiting NF-κB activities. Sci Rep. 5:11822. DOI: 10.1038/srep11822. PMID: 26149595. PMCID: PMC4493574.27. Xu L, Li J, Tang X, Wang Y, Ma Z, Gao Y. 2019; Metabolomics of aurantio-obtusin-induced hepatotoxicity in rats for discovery of potential biomarkers. Molecules. 24:3452. DOI: 10.3390/molecules24193452. PMID: 31547563. PMCID: PMC6804130. PMID: f8b1f1475f374e058ef6ce7934b928c5.28. Rong S, Park JK, Kirsch T, Yagita H, Akiba H, Boenisch O, Haller H, Najafian N, Habicht A. 2011; The TIM-1:TIM-4 pathway enhances renal ischemia-reperfusion injury. J Am Soc Nephrol. 22:484–495. DOI: 10.1681/ASN.2010030321. PMID: 21355054. PMCID: PMC3060442.29. Ronco C, Bellomo R, Kellum JA. 2019; Acute kidney injury. Lancet. 394:1949–1964. DOI: 10.1016/S0140-6736(19)32563-2. PMID: 31777389.30. Ronco C. 2014; Lipopolysaccharide (LPS) from the cellular wall of Gram-negative bacteria, also known as endotoxin, is a key molecule in the pathogenesis of sepsis and septic shock. Preface. Blood Purif. 37 Suppl 1:1. DOI: 10.1159/000356831. PMID: 24457488.31. Zarjou A, Agarwal A. 2011; Sepsis and acute kidney injury. J Am Soc Nephrol. 22:999–1006. DOI: 10.1681/ASN.2010050484. PMID: 21566052.32. van der Poll T, Meijers JC. 2010; Systemic inflammatory response syndrome and compensatory anti-inflammatory response syndrome in sepsis. J Innate Immun. 2:379–380. DOI: 10.1159/000318190. PMID: 20606408.33. Zhou F, Ding M, Gu Y, Fan G, Liu C, Li Y, Sun R, Wu J, Li J, Xue X, Li H, Li X. 2022; Aurantio-obtusin attenuates non-alcoholic fatty liver disease through AMPK-mediated autophagy and fatty acid oxidation pathways. Front Pharmacol. 12:826628. DOI: 10.3389/fphar.2021.826628. PMID: 35087411. PMCID: PMC8787202. PMID: ec8e59eacb7748d39749f85ef683b064.34. Guo CY, Liao WT, Qiu RJ, Zhou DS, Ni WJ, Yu CP, Zeng Y. 2021; Aurantio-obtusin improves obesity and insulin resistance induced by high-fat diet in obese mice. Phytother Res. 35:346–360. DOI: 10.1002/ptr.6805. PMID: 32749748.35. Li YJ, Wu RY, Liu RP, Wu KY, Ding MN, Sun R, Gu YQ, Zhou F, Wu JZ, Zheng Q, Duan SN, Li RR, Zhang YH, Li FH, Li X. 2023; Aurantio-obtusin ameliorates obesity by activating PPARα-dependent mitochondrial thermogenesis in brown adipose tissues. Acta Pharmacol Sin. 44:1826–1840. DOI: 10.1038/s41401-023-01089-4. PMID: 37095199.36. Paudel P, Kim DH, Jeon J, Park SE, Seong SH, Jung HA, Choi JS. 2021; Neuroprotective effect of aurantio-obtusin, a putative vasopressin V1A receptor antagonist, on transient forebrain ischemia mice model. Int J Mol Sci. 22:3335. DOI: 10.3390/ijms22073335. PMID: 33805177. PMCID: PMC8037569. PMID: 5b5ad1f1ed114e5ba03764cfc146575f.37. Hu M, Lin L, Liu J, Zhong Y, Liang B, Huang Y, Li Z, Lin X, Wang B, Zhang B, Meng H, Ye R, Du J, Dai M, Peng Y, Li H, Wu Q, Gao H, Yang X, Huang Z. 2022; Aurantio-obtusin induces hepatotoxicity through activation of NLRP3 inflammasome signaling. Toxicol Lett. 354:1–13. DOI: 10.1016/j.toxlet.2021.10.011. PMID: 34718095.38. Cai D, Duan H, Fu Y, Cheng Z. 2021; Renal tissue damage induced by acute kidney injury in sepsis rat model is inhibited by cynaropicrin via IL-1β and TNF-α down-regulation. Dokl Biochem Biophys. 497:151–157. DOI: 10.1134/S1607672921020022. PMID: 33895932.39. Deng P, Tang N, Li L, Zou G, Xu Y, Liu Z. 2022; Diagnostic value of combined detection of IL-1β, IL-6, and TNF-α for sepsis-induced cardiomyopathy. Med Clin (Barc). 158:413–417. DOI: 10.1016/j.medcli.2021.04.025. PMID: 34147250.40. Qin W, Yang Z, Yin J, Chen D, Huo J, Wang J, Wang L, Zhuo Q. 2022; Effect assessment of aurantio-obtusin on novel human renal glomerular endothelial cells model using a microfluidic chip. Nutrients. 14:4615. DOI: 10.3390/nu14214615. PMID: 36364876. PMCID: PMC9654768. PMID: 8ee0c8734ca6488c9dae4828a5587be6.41. Wu H, Liu J, Li W, Liu G, Li Z. 2016; LncRNA-HOTAIR promotes TNF-α production in cardiomyocytes of LPS-induced sepsis mice by activating NF-κB pathway. Biochem Biophys Res Commun. 471:240–246. DOI: 10.1016/j.bbrc.2016.01.117. PMID: 26806307.42. Lv H, Tian M, Hu P, Wang B, Yang L. 2021; Overexpression of miR-365a-3p relieves sepsis-induced acute myocardial injury by targeting MyD88/NF-κB pathway. Can J Physiol Pharmacol. 99:1007–1015. DOI: 10.1139/cjpp-2020-0646. PMID: 33852805.43. Han SJ, Kim M, D'Agati VD, Lee HT. 2019; 6-Shogaol protects against ischemic acute kidney injury by modulating NF-κB and heme oxygenase-1 pathways. Am J Physiol Renal Physiol. 317:F743–F756. DOI: 10.1152/ajprenal.00182.2019. PMID: 31313953. PMCID: PMC6766624.44. Shi M, Zeng X, Guo F, Huang R, Feng Y, Ma L, Zhou L, Fu P. 2017; Anti-inflammatory pyranochalcone derivative attenuates LPS-induced acute kidney injury via inhibiting TLR4/NF-κB pathway. Molecules. 22:1683. DOI: 10.3390/molecules22101683. PMID: 28994737. PMCID: PMC6151422. PMID: 5ec9c90c34854bd0a4b599b17a48aa29.45. Kay J, Thadhani E, Samson L, Engelward B. 2019; Inflammation-induced DNA damage, mutations and cancer. DNA Repair (Amst). 83:102673. DOI: 10.1016/j.dnarep.2019.102673. PMID: 31387777. PMCID: PMC6801086.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Wnt-C59 inhibits proinflammatory cytokine expression by reducing the interaction between β-catenin and NF-κB in LPS-stimulated epithelial and macrophage cells
- Viridicatol from Marine-derived Fungal Strain Penicillium sp. SF-5295 Exerts Anti-inflammatory Effects through Inhibiting NF-kappaB Signaling Pathway on Lipopolysaccharide-induced RAW264.7 and BV2 Cells
- Neogambogic acid relieves myocardial injury induced by sepsis via p38 MAPK/NF-κB pathway
- Aromadendrin Inhibits Lipopolysaccharide-Induced Nuclear Translocation of NF-kappaB and Phosphorylation of JNK in RAW 264.7 Macrophage Cells
- Protective effects and mechanism of coenzyme Q10 and vitamin C on doxorubicin-induced gastric mucosal injury and effects of intestinal flora