Blood Res.
2023 Dec;58(4):181-186. 10.5045/br.2023.2023152.
Treatment outcome and prognostic factors in relapsed pediatric acute myeloid leukemia
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Health Science and Technology, SAIHST, Sungkyunkwan University School of Medicine, Seoul, Korea
- 3Cell & Gene Therapy Institute, Samsung Medical Center, Seoul, Korea
- KMID: 2550195
- DOI: http://doi.org/10.5045/br.2023.2023152
Abstract
- Background
Despite improved outcomes for pediatric patients with acute myeloid leukemia (AML), the prognosis for relapse remains poor. This study aimed to examine the clinical factors associated with prognosis in relapsed pediatric AML.
Methods
We conducted a chart review of pediatric patients with AML who experienced their first relapse and received treatment at our institution between 2008 and 2019. Risk stratification at diagnosis was performed according to the definition suggested by the ongoing AML 2012 study in Korea, and the clinical factors associated with prognosis were analyzed.
Results
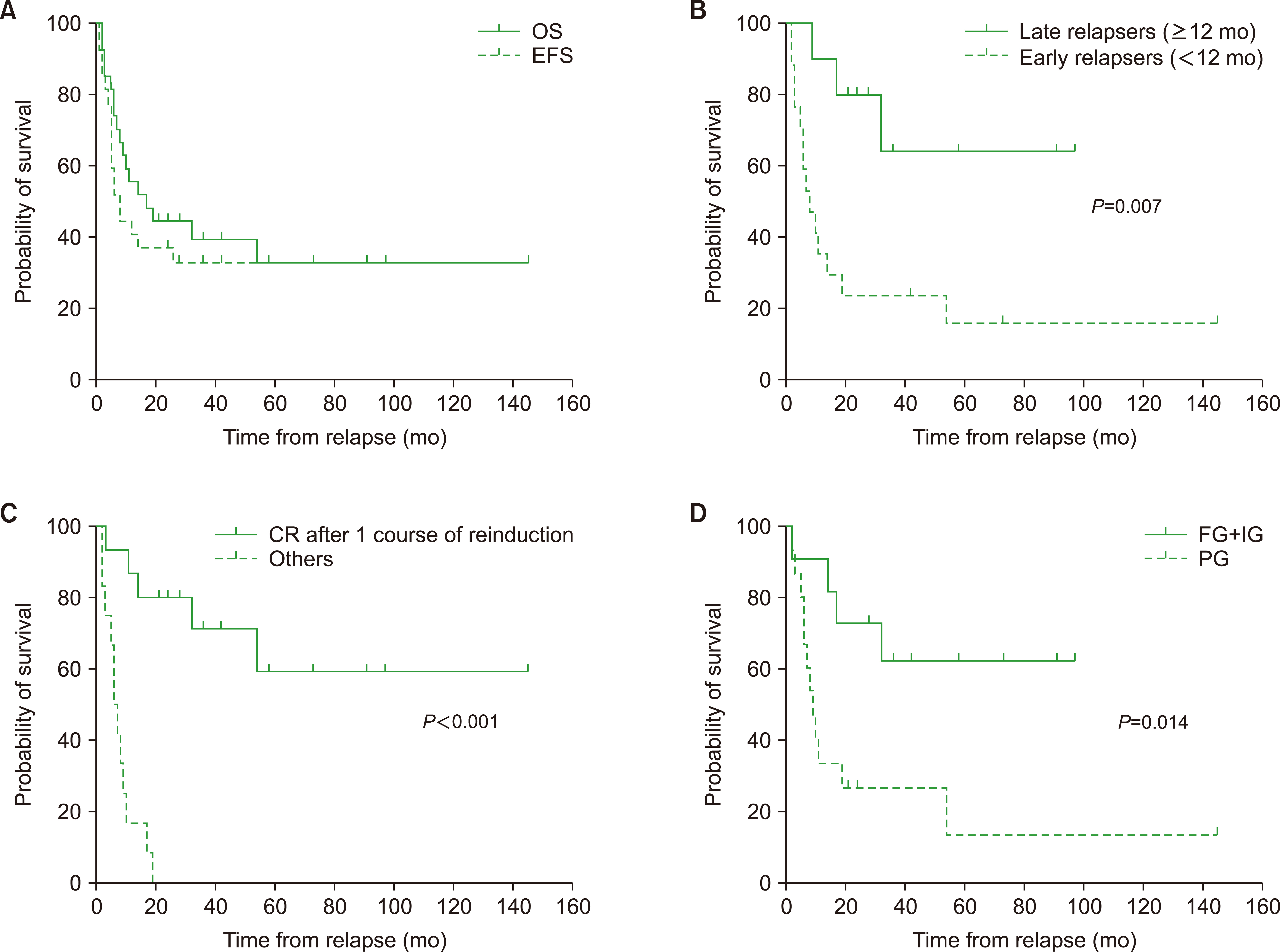
A total of 27 pediatric patients with relapsed AML were identified. The 5-year overall survival (OS) and event-free survival (EFS) rates were 32.9% and 32.9%, respectively. A duration ≥12 months from diagnosis to relapse had a favorable impact on survival outcomes (5-yr OS, 64.0% vs. 15.7%; P =0.007). Patients who achieved complete remission (CR) after 1 course of chemotherapy following relapse (N=15) had a 5-year OS rate of 59.3%, while none of the other patients survived (P<0.0001). Additionally, the 5-year OS differed significantly based on the risk group at initial diagnosis (62.3% [favorable and intermediate prognosis groups, N=11] vs. 13.3% [poor prognosis group, N=15]; P=0.014).
Conclusion
Patients with a longer duration of CR before relapse, who achieved CR following 1 course of reinduction chemotherapy, and were in the favorable or intermediate prognosis group at diagnosis demonstrated better outcomes. These findings emphasize the importance of tailoring treatment strategies based on the expected prognosis at relapse in pediatric patients with AML.
Keyword
Figure
Reference
-
1. Park HJ, Moon EK, Yoon JY, et al. 2016; Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 48:869–82. DOI: 10.4143/crt.2015.290. PMID: 26790965. PMCID: PMC4946351.2. Puumala SE, Ross JA, Aplenc R, Spector LG. 2013; Epidemiology of childhood acute myeloid leukemia. Pediatr Blood Cancer. 60:728–33. DOI: 10.1002/pbc.24464. PMID: 23303597. PMCID: PMC3664189.3. Abrahamsson J, Forestier E, Heldrup J, et al. 2011; Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 29:310–5. DOI: 10.1200/JCO.2010.30.6829. PMID: 21149663.4. Rubnitz JE, Inaba H, Dahl G, et al. 2010; Minimal residual disease- directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 11:543–52. DOI: 10.1016/S1470-2045(10)70090-5. PMID: 20451454.5. Tsukimoto I, Tawa A, Horibe K, et al. 2009; Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 27:4007–13. DOI: 10.1200/JCO.2008.18.7948. PMID: 19620491.6. Creutzig U, Zimmermann M, Bourquin JP, et al. 2013; Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 122:37–43. DOI: 10.1182/blood-2013-02-484097. PMID: 23704089.7. Rubnitz JE, Lacayo NJ, Inaba H, et al. 2019; Clofarabine can replace anthracyclines and etoposide in remission induction therapy for childhood acute myeloid leukemia: the AML08 multicenter, randomized phase III trial. J Clin Oncol. 37:2072–81. DOI: 10.1200/JCO.19.00327. PMID: 31246522. PMCID: PMC7001777.8. Gamis AS, Alonzo TA, Meshinchi S, et al. 2014; Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. 32:3021–32. DOI: 10.1200/JCO.2014.55.3628. PMID: 25092781. PMCID: PMC4162498.9. Pession A, Masetti R, Rizzari C, et al. 2013; Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 122:170–8. DOI: 10.1182/blood-2013-03-491621. PMID: 23673857.10. Burnett AK, Hills RK, Milligan DW, et al. 2010; Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol. 28:586–95. DOI: 10.1200/JCO.2009.22.9088. PMID: 20038732.11. Gorman MF, Ji L, Ko RH, et al. 2010; Outcome for children treated for relapsed or refractory acute myelogenous leukemia (rAML): a Therapeutic Advances in Childhood Leukemia (TACL) Consortium study. Pediatr Blood Cancer. 55:421–9. DOI: 10.1002/pbc.22612. PMID: 20658611.12. Creutzig U, Zimmermann M, Dworzak MN, et al. 2014; The prognostic significance of early treatment response in pediatric relapsed acute myeloid leukemia: results of the international study relapsed AML 2001/01. Haematologica. 99:1472–8. DOI: 10.3324/haematol.2014.104182. PMID: 24763401. PMCID: PMC4562536.13. Moritake H, Tanaka S, Miyamura T, et al. 2021; The outcomes of relapsed acute myeloid leukemia in children: results from the Japanese Pediatric Leukemia/Lymphoma Study Group AML-05R study. Pediatr Blood Cancer. 68:e28736. DOI: 10.1002/pbc.28736. PMID: 32991072.14. Rasche M, Steidel E, Zimmermann M, et al. 2021; Second relapse of pediatric patients with acute myeloid leukemia: a report on current treatment strategies and outcome of the AML-BFM Study Group. Cancers (Basel). 13:789. DOI: 10.3390/cancers13040789. PMID: 33672815. PMCID: PMC7918758. PMID: 1baebca711d84d67a51adec0cb9cf17a.15. van Eijkelenburg NKA, Rasche M, Ghazaly E, et al. 2018; Clofarabine, high-dose cytarabine and liposomal daunorubicin in pediatric relapsed/refractory acute myeloid leukemia: a phase IB study. Haematologica. 103:1484–92. DOI: 10.3324/haematol.2017.187153. PMID: 29773602. PMCID: PMC6119144.16. Ramaswamy K, Steinherz PG, Agrawal AK, et al. 2022; Clofarabine with topotecan, vinorelbine, and thiotepa reinduction regimen for children and young adults with relapsed AML. Blood Adv. 6:2688–94. DOI: 10.1182/bloodadvances.2021005753. PMID: 35008101. PMCID: PMC9043926.17. Brivio E, Baruchel A, Beishuizen A, et al. 2022; Targeted inhibitors and antibody immunotherapies: novel therapies for paediatric leukaemia and lymphoma. Eur J Cancer. 164:1–17. DOI: 10.1016/j.ejca.2021.12.029. PMID: 35121370.18. Niktoreh N, Lerius B, Zimmermann M, et al. 2019; Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. 104:120–7. DOI: 10.3324/haematol.2018.191841. PMID: 30093401. PMCID: PMC6312035.19. Dhunputh C, Strullu M, Petit A, et al. 2022; Single-dose (4.5 mg/m2) gemtuzumab ozogamicin in combination with fludarabine, cytarabine and anthracycline as reinduction therapy in relapsed or refractory paediatric acute myeloid leukaemia. Br J Haematol. 198:373–81. DOI: 10.1111/bjh.18203. PMID: 35438187.20. Kaspers GJ, Zimmermann M, Reinhardt D, et al. 2013; Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. 31:599–607. DOI: 10.1200/JCO.2012.43.7384. PMID: 23319696.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advances in the treatment of pediatric acute leukemia
- Outcome of Intensive Therapy for Children with Relapsed Acute Myeloid Leukemia: A Single Institution Korean Study
- Treatments for children and adolescents with AML
- Current treatment for pediatric acute myeloid leukemia
- Prognostic factors and treatment of pediatric acute lymphoblastic leukemia